Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction?
ABSTRACT
IBS is one of the most common types of functional bowel disorder. Increasing attention has been paid to the causative role of food in IBS. Food ingestion precipitates or exacerbates symptoms, such as abdominal pain and bloating in patients with IBS through different hypothesised mechanisms including immune and mast cell activation, mechanoreceptor stimulation and chemosensory activation. Wheat is regarded as one of the most relevant IBS triggers, although which component(s) of this cereal is/are involved remain(s) unknown. Gluten, other wheat proteins, for example, amylase-trypsin inhibitors, and fructans (the latter belonging to fermentable oligo-di-mono-saccharides and polyols (FODMAPs)), have been identified as possible factors for symptom generation/exacerbation. This uncertainty on the true culprit(s) opened a scenario of semantic definitions favoured by the discordant results of double-blind placebo-controlled trials, which have generated various terms ranging from non-coeliac gluten sensitivity to the broader one of non-coeliac wheat or wheat protein sensitivity or, even, FODMAP sensitivity. The role of FODMAPs in eliciting the clinical picture of IBS goes further since these short-chain carbohydrates are found in many other dietary components, including vegetables and fruits. In this review, we assessed current literature in order to unravel whether gluten/wheat/FODMAP sensitivity represent ‘facts’ and not ‘fiction’ in IBS symptoms. This knowledge is expected to promote standardisation in dietary strategies (gluten/wheat-free and low FODMAP) as effective measures for the management of IBS symptoms.
Extract from study:
WHEAT SENSITIVITY
Wheat is considered one of the foods known to evoke IBS symptoms. However, which component(s) of wheat is/are actually responsible for these clinical effects still remain(s) an unsettled issue. The two parts of wheat that are thought to have a mechanistic effect comprise proteins (primarily, but not exclusively, gluten) and carbohydrates (primarily indigestible short-chain components, FODMAPs). Two distinct views characterise the clinical debate: one line identifies wheat proteins as a precipitating/perpetuating factor leading to symptoms, while the other believes that FODMAPs are the major trigger for IBS.
The controversy over nomenclature
If gluten is a major trigger for IBS, it expands the gluten-related disorders by adding a new entity now referred to as non-coeliac gluten sensitivity (NCGS). Indeed, coeliac disease-like abnormalities were reported in a subgroup of patients with IBS many years ago. A recent expert group of researchers reached unanimous consensus attesting the existence of a syndrome triggered by gluten ingestion. This syndrome recognises a wide spectrum of symptoms and manifestations including an IBS-like phenotype, along with an extra-intestinal phenotype, that is, malaise, fatigue, headache, numbness, mental confusion (‘brain fog’), anxiety, sleep abnormalities, fibromyalgia-like symptoms and skin rash. In addition, other possible clinical features include gastroesophageal reflux disease, aphthous stomatitis, anaemia, depression, asthma and rhinitis. Symptoms or other manifestations occur shortly after gluten consumption and disappear or recur in a few hours (or days) after gluten withdrawal or challenge. A fundamental prerequisite for suspecting NCGS is to rule out all the established gluten/wheat disorders, comprising coeliac disease (CD), gluten ataxia, dermatitis herpetiformis and wheat allergy. The major issue not addressed by the consensus opinion was that gluten is only one protein contained within wheat. Other proteins, such as amylase-trypsin inhibitors (ATIs), are strong activators of innate immune responses in monocytes, macrophages and dendritic cells. Furthermore, wheat germ agglutinin, which has epithelial-damaging and immune effects at very low doses at least in vitro, might also contribute to both intestinal and extraintestinal manifestations of NCGS. Consequently, a further development of this research field led to suggestions of a broader term, non-coeliac wheat sensitivity (NCWS). The problems with this term are twofold. First, rye and barley may be inappropriately excluded. Second, the term will refer to any wheat component that might be causally related to induction of symptoms and, therefore, will also include fructans (FODMAPs). It will then have a very nonspecific connotation in IBS. A more correct term would then be non-coeliac wheat protein sensitivity (NCWPS) since this does not attribute effects to gluten without evidence of such specificity, eliminates the issue of fructan-induced symptoms and avoids the unknown contribution of rye and barley proteins to the symptoms. Both NCGS, the currently accepted term, and NCWPS will be used subsequently in this paper.
Monococcum wheat (einkorn wheat): why it is so important
Summary of the main characteristics of the monococcum wheat (einkorn) which give it great potential to be used for the preparation of bakery products but also sweet ones for people who:
1. are genetically predisposed for celiac disease (1) (2) (3) (4) (5),
2. must keep the glycemic index under control (6),
3. are non-celiac gluten sensitive, reintroduce gluten after its exclusion (7),
4. have difficulty digesting gluten (8).
5. are sensitive to ATI -amylase trypsina inhibitors-. (9)
6. Also worthy of note is the high nutritional qualities of monococcus wheat (einkorn) (10)
(1)- Immunogenicity of monococcum wheat in celiac patients
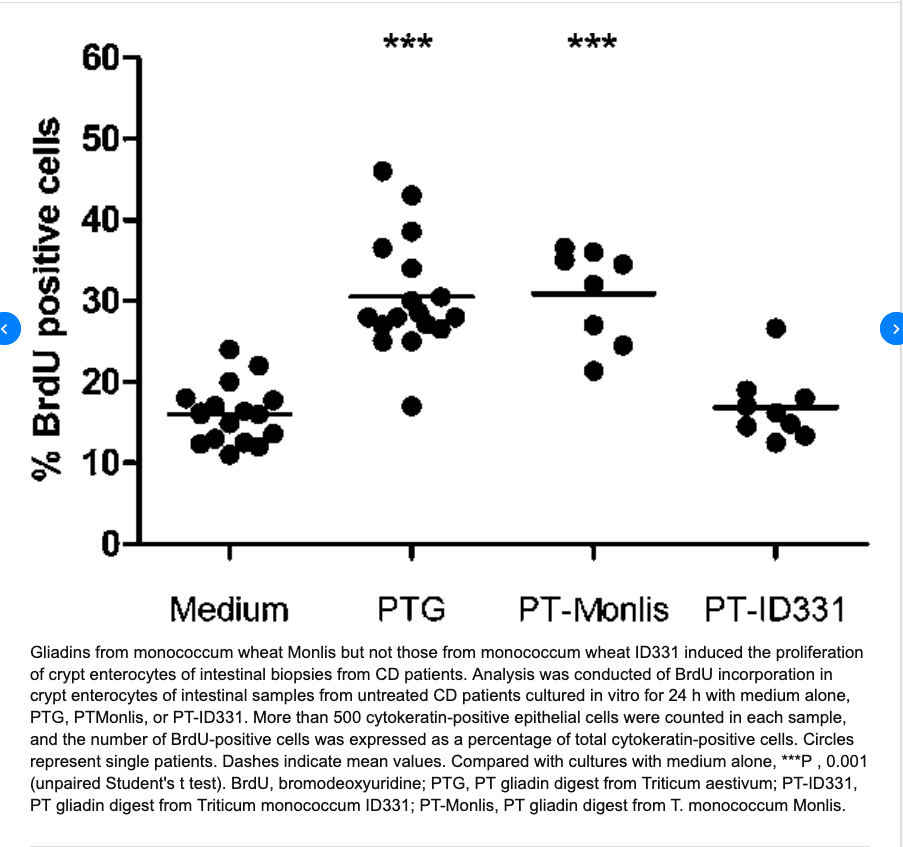
………..omissis. “Conclusions: Our data show that the monococcum lines Monlis and ID331 activate the CD T cell response and suggest that these lines are toxic for celiac patients. However, ID331 is likely to be less effective in inducing CD because of its inability to activate the innate immune pathways”. Immunogenicity of monococcum wheat in celiac patients. Carmen Gianfrani et altri. Am J Clin Nutr 2012;96:1339–45.
(2) ………omissis. “D’altra parte, tenuto conto che l’incidenza e la gravità della celiachia dipende dalla quantità e dalla nocività delle prolamine e che alcuni genotipi di grano monococco hanno una elevata qualità panificatoria accoppiata con assenza di citotossicità e ridotta immunogenicità, è atteso che l’uso delle farine di monococco nella dieta della popolazione generale, all’interno della quale si trova una elevata percentuale di individui predisposti geneticamente alla celiachia ma non ancora celiaci, possa contribuire a contenere la diffusione di questa forma di intolleranza alimentare. Ciò lascia pensare che il grano monococco, riportato recentemente in coltivazione in Italia dai ricercatori del Consiglio per la Ricerca e la sperimentazione in Agricoltura (CRA) di Roma e San Angelo Lodigiano, potrà svolgere un ruolo importante nella prevenzione della celiachia, sia direttamente sotto forma di pane e pasta sia indirettamente come specie modello per lo studio del ruolo dell’immunità innata nell’insorgenza della celiachia”. Le nuove frontiere delle tecnologie alimentari e la celiachia Norberto Pogna, Laura Gazza (2013).
(3)-Extensive in vitro gastrointestinal digestion markedly reduces the immune-toxicity of Triticum monococcum wheat: Implication for celiac disease
Carmen Gianfrani, Alessandra Camarca, Giuseppe Mazzarella, Luigia Di Stasio, Nicola Giardullo, Pasquale Ferranti, Gianluca Picariello, Vera Rotondi Aufiero, Stefania Picascia, Riccardo Troncone, Norberto Pogna, Salvatore Auricchio
and Gianfranco Mamone. Mol. Nutr. Food Res. 2015, 00, 1–11
Scope: The ancient diploid Triticum monococcum is of special interest as a candidate low-toxic wheat species for celiac disease patients. Here, we investigated how an in vitro gastro-intestinal digestion, affected the immune toxic properties of gliadin from diploid compared to hexaploid wheat.
Method and results: Gliadins from Triticum monococcum, and Triticum aestivum cultivars were digested using either a partial proteolysis with pepsin-chymotrypsin, or an extensive degradation that used gastrointestinal enzymes including the brush border membrane enzymes. The immune stimulatory properties of the digested samples were investigated on T-cell lines and jejunal biopsies from celiac disease patients. The T-cell response profile to the Triticum mono coccum gliadin was comparable to that obtained with Triticum aestivum gliadin after the partial pepsin-chymotrypsin digestion. In contrast, the extensive gastrointestinal hydrolysis drastically reduced the immune stimulatory properties of Triticum monococcum gliadin. MS-based analy- sis showed that several Triticum monococcum peptides, including known T-cell epitopes, were degraded during the gastrointestinal treatment, whereas many of Triticum aestivum gliadin survived the gastrointestinal digestion.
Conclusion: he pattern of Triticum monococcum gliadin proteins is sufficiently different from those of common hexaploid wheat to determine a lower toxicity in celiac disease patients following in vitro simulation of human digestion.
The Effect of Digestion and Digestibility on Allergenicity of Food
The Effect of Digestion and Digestibility on Allergenicity of Food(second part)
From the chapter: “Digestion of Proteins: Gastric Acid is Critical for Adequate Protein Digestion and Prevention of Food Allergy
Digestion of proteins -and therefore most food allergens- is initiated in the stomach. A low pH is essential for the inactive enzyme pepsinogen to get activated into pepsin [92]. However, if acid-suppressing drugs are given, the pH increases considerably (e.g., up to 5 with proton pump inhibitors, PPI). As shown in many previous in vitro experiments, the proper digestion by pepsin is hindered when the pH is increased (Figure 1), and this is true for a number of food proteins, like hazelnut[93], codfish [94], milk [95], and casein (Figure 1).
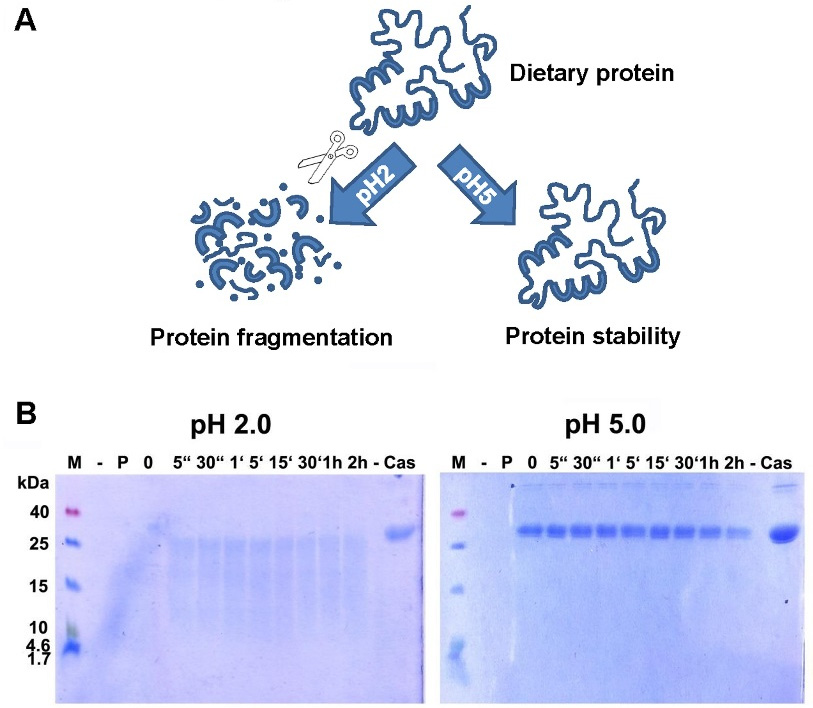
(A) Digestion of proteins is hampered when pH increases. Proteins, as part of the daily diet, are digested at low pH and broken down into smaller fragments, whereas a higher pH blocks proper digestion. The resulting bigger fragments or proteins are more easily recognized by the immune system, leading to an increased risk for sensitization or allergic reactions. (B) Digestion of α-casein in vitro is hampered when pH increases. Casein was readily broken down by enzymatic digestion with pepsin at pH 2.0, but remained totally intact even after 2 h of incubation with enzyme at pH 5.0. M: molecular weight marker; -: empty lane; P: pepsin; 0: no incubation time, reaction stopped immediately; “: seconds; ‘: minutes; h: hour(s); Cas: casein.
It is clear that food intake per se changes the gastric pH, which can increase from a median fasting baseline value of pH 1 to pH 4.5 with ingestion of the meal [96]. The buffer capacity thereby depends on the food composition and meal constituents. However, this effect is transient, as ongoing acid production is responsible for a subsequent decrease of the pH, which returns to ca. pH 1 about 260 min after the start of the meal [96]. Applying acid-suppressing substances can disturb this process and induce a long-lasting elevation of the gastric pH up to 5.0 [97]. In a number of food animal models, the effect of this pH-elevation was shown in vivo, as feeding digestion-labile antigen under concomitant acid-suppression resulted in a clear Th2-response and allergy symptoms [98,99,100,101,102,103,104]. This acquired sensitization capacity was true for different proteins, like codfish, hazelnut or ovalbumin, and even oral drugs, in the mouse model [99] and also in humans [105]. Importantly, several types of acid-suppressing or -neutralizing medication, like base powder [106], sucralfate [102], H2-receptor blockers [107] and proton pump inhibitors [101] produced this effect. The outcome of the immune response may depend on timing of the anti-acid drug application in relation to food uptake, and on the dosage of the antigen [101,108]. Gastric acid suppression might further impact on intestinal pH levels and consequently on protein digestion in the intestine [109]. This assumption, however, requires further investigations in clinical settings.” “The Effect of Digestion and Digestibility on Allergenicity of Food Isabella Pali-Scholl, Eva Untersmayr, Martina Klems and Erika Jensen-Jarolim. Published: 21 August 2018 Nutrients.”
The effect of digestion and digestibily on allergenicity of food (First part)
Deepening
The Effect of Digestion and Digestibility on Allergenicity of Food
