The Effect of Digestion and Digestibility on Allergenicity of Food(second part)
From the chapter: “Digestion of Proteins: Gastric Acid is Critical for Adequate Protein Digestion and Prevention of Food Allergy
Digestion of proteins -and therefore most food allergens- is initiated in the stomach. A low pH is essential for the inactive enzyme pepsinogen to get activated into pepsin [92]. However, if acid-suppressing drugs are given, the pH increases considerably (e.g., up to 5 with proton pump inhibitors, PPI). As shown in many previous in vitro experiments, the proper digestion by pepsin is hindered when the pH is increased (Figure 1), and this is true for a number of food proteins, like hazelnut[93], codfish [94], milk [95], and casein (Figure 1).
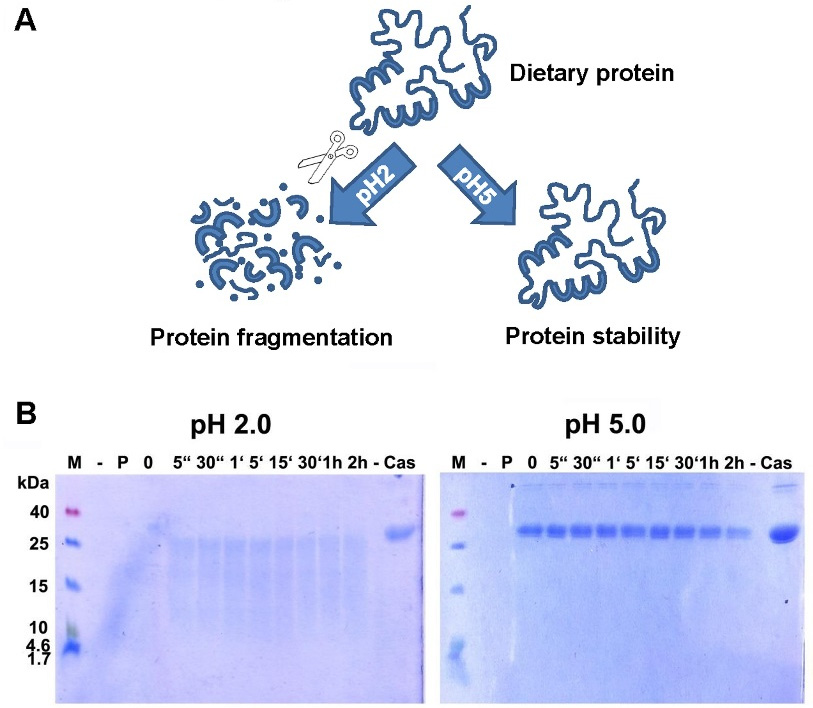
(A) Digestion of proteins is hampered when pH increases. Proteins, as part of the daily diet, are digested at low pH and broken down into smaller fragments, whereas a higher pH blocks proper digestion. The resulting bigger fragments or proteins are more easily recognized by the immune system, leading to an increased risk for sensitization or allergic reactions. (B) Digestion of α-casein in vitro is hampered when pH increases. Casein was readily broken down by enzymatic digestion with pepsin at pH 2.0, but remained totally intact even after 2 h of incubation with enzyme at pH 5.0. M: molecular weight marker; -: empty lane; P: pepsin; 0: no incubation time, reaction stopped immediately; “: seconds; ‘: minutes; h: hour(s); Cas: casein.
It is clear that food intake per se changes the gastric pH, which can increase from a median fasting baseline value of pH 1 to pH 4.5 with ingestion of the meal [96]. The buffer capacity thereby depends on the food composition and meal constituents. However, this effect is transient, as ongoing acid production is responsible for a subsequent decrease of the pH, which returns to ca. pH 1 about 260 min after the start of the meal [96]. Applying acid-suppressing substances can disturb this process and induce a long-lasting elevation of the gastric pH up to 5.0 [97]. In a number of food animal models, the effect of this pH-elevation was shown in vivo, as feeding digestion-labile antigen under concomitant acid-suppression resulted in a clear Th2-response and allergy symptoms [98,99,100,101,102,103,104]. This acquired sensitization capacity was true for different proteins, like codfish, hazelnut or ovalbumin, and even oral drugs, in the mouse model [99] and also in humans [105]. Importantly, several types of acid-suppressing or -neutralizing medication, like base powder [106], sucralfate [102], H2-receptor blockers [107] and proton pump inhibitors [101] produced this effect. The outcome of the immune response may depend on timing of the anti-acid drug application in relation to food uptake, and on the dosage of the antigen [101,108]. Gastric acid suppression might further impact on intestinal pH levels and consequently on protein digestion in the intestine [109]. This assumption, however, requires further investigations in clinical settings.” “The Effect of Digestion and Digestibility on Allergenicity of Food Isabella Pali-Scholl, Eva Untersmayr, Martina Klems and Erika Jensen-Jarolim. Published: 21 August 2018 Nutrients.”
The effect of digestion and digestibily on allergenicity of food (First part)
Deepening
The Effect of Digestion and Digestibility on Allergenicity of Food
Gluten: amino acids, digestion, toxic peptides
Gliadin and Glutenin
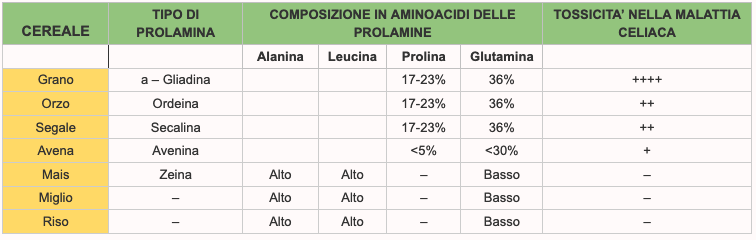
They are the wheat proteins (gliadin, soluble in alcohol and glutenin, insoluble in alcohol) and are composed of amino acid chains (1). Gliadin is made up of the union of about 100-200 amino acids (the main cause of celiac disease), and glutenin, consisting of a combination of about 2,000-20,000 amino acids. The covalent bond that unites two amino acids also takes the name in biochemistry of “peptide bond”. A chain of multiple amino acids linked through peptide bonds takes the generic name of peptide or polypeptide or oligopeptide if the number of amino acids involved is limited; one or more polypeptides, sometimes accompanied by other auxiliary structures or ions called cofactors or prosthetic groups, constitute a protein. amino-acids (or amminoacids) are the primary structural unit of proteins. We can therefore imagine the amino acids as bricks that, united by a glue called peptide bond, form a long sequence that gives rise to a protein. Alcohol soluble cereal proteins are called: prolamines.
The wheat prolamine is gliadin, that of barley is hordein; that of rye is secaline, that of avena is avenin. The different types of prolamins contain different amino-acids and the higher the content of proline and glutamine (which are some of the amino-acids that compose it) the more the prolamine, and therefore the peptides of that cereal will be toxic (2) for the affected patient from celiac disease. The highest levels of proline and glutamine are in wheat, barley and rye. Also glutenins have some toxic sequences for celiacs but they appear to be much less active in soliciting the adverse response of the humanitarian system of man.