6 Exercise as a regulator of intestinal barrier integrity
Regular moderate physical exercises are one of the most common recommendations for the prevention of various pathologies, including disruption of the integrity of the intestinal barrier. This may be due to the influence of the gut microbiota. In particular, exercises have been found to increase gut bacterial diversity (Hintikka et al., 2023). However, effects of physical exercises depend on their intensity. For example, endurance athletes have a high incidence of gastrointestinal disorders and the “leaky” gut is one of the most common disorders (Ribeiro et al., 2021). It is characterized by dysfunction of the intestinal epithelial barrier and its excessive permeability. This results in penetration of harmful microorganisms, toxins or undigested food particles into the bloodstream and has a negative effect on health of the whole organism (Aleman et al., 2023).
The effect of exercise on intestinal permeability depends on its duration and intensity. For example, people who exercise frequently and intensely have the same mortality rates as people who lead a sedentary lifestyle (Van Houten et al., 2015). A 60 min bout of intensive treadmill running increased the permeability of the small intestine in runners, whereas low-intensity running had no such effect (Pals et al., 1997). Using the overtraining model with male C57BL/6 mice, it was established that exhaustive exercise exacerbated intestinal inflammation, disrupted integrity and enhanced intestine wall permeability (Hou et al., 2020). Sustained strenuous exercise in racing sled dogs increased the intestinal permeability and the frequency of gastric erosions or ulcerations (Davis et al., 2005). High-intensity interval running increased intestine wall permeability and intestinal-fatty acid binding protein (I-FABP) release in male runners (Pugh et al., 2017). I-FABP is a cytoplasmic protein expressed exclusively in the enterocytes of the small intestine and its increased concentration in the blood is used as a marker of damage to intestinal epithelial cells (Sikora et al., 2019).
Physical exercise of low/moderate intensity can often have positive effects and can be considered as a method of non-pharmacological intervention in inflammatory bowel disease (Ordille and Phadtare, 2023). For example, mice that swam for 30 min before inducing intestinal barrier dysfunction had less intestinal dysfunction compared to mice that had not swum before. This might happen due to a strengthening of antimicrobial function of the intestine as a result of the increase in expression of antimicrobial peptides (Luo et al., 2014). Obese mice that were trained on a motorized treadmill for 45 min per day 5 days a week for 12 weeks had higher expression levels of colonic ZO-1 and occludin. Moderate exercise effectively prevented the development of dysbacteriosis caused by the HFD, as well as intestinal pathology (Wang et al., 2022). Dysbacteriosis and impaired intestinal barrier integrity induced by HFD in wild type mice was prevented by exercise. Exercise on a motor-driven rodent treadmill for 5 days a week for a total of 15 weeks significantly reversed the pathological changes. Ablation of Sestrin 2 protein attenuated the protective effects of exercise, suggesting its involvement in regulation of intestinal permeability (Yu et al., 2022). Thus, it can be concluded that high-intensity exercises often have a negative effect on the integrity of the intestine, whereas low- and moderate-intensity regular exercise can have a positive effects. It may be speculated that moderate damage to the intestinal wall is a hormetic factor that may be used to train organisms to cope with severe damaging challenges. This may be used to increase the adaptive potential of organisms to prevent damaging effects of any stresses of physical and chemical nature on the integrity of the intestinal wall.
6.1 Exercise-induced heat stress
It is known that physical exertion causes heat stress and associated dysfunction of gut integrity. A systematic review examining the relationship between an exercise-induced increase in core body temperature and intestinal permeability demonstrated that the magnitude of exercise-induced hyperthermia correlated with increased intestinal permeability (Pires et al., 2017). An increase in body temperature is a signal to activate the expression of heat shock proteins (HSP) which constitutively function as molecular chaperones maintaining the native structure of the proteins. Their expression is mainly triggered by heat shock signals. During exercise, the level of HSP70 and HSP90 increase (Krüger et al., 2019). Expression of HSP is regulated at the level of heat shock factors (HSF) such as HSF1 that is expressed in all mammalian tissues. Normally it resides in the cytoplasm as a monomer. In response to stressful conditions, it trimerizes, translocates into the nucleus, binds to the heat shock element of target genes and activates the transcription of HSPs, including HSP70/90 (Noble and Shen, 2012).
In this way, exercises cause a homeostatic imbalance, while regular training is adaptive and decreases the degree of this imbalance. Potentially, a higher adaptive steady-state level of HSPs due to regular training could explain their positive effect on gut integrity. At that time, during acute physical exertion, HSPs probably cannot cope with that level of homeostatic imbalance caused exercise-induced heat stress.
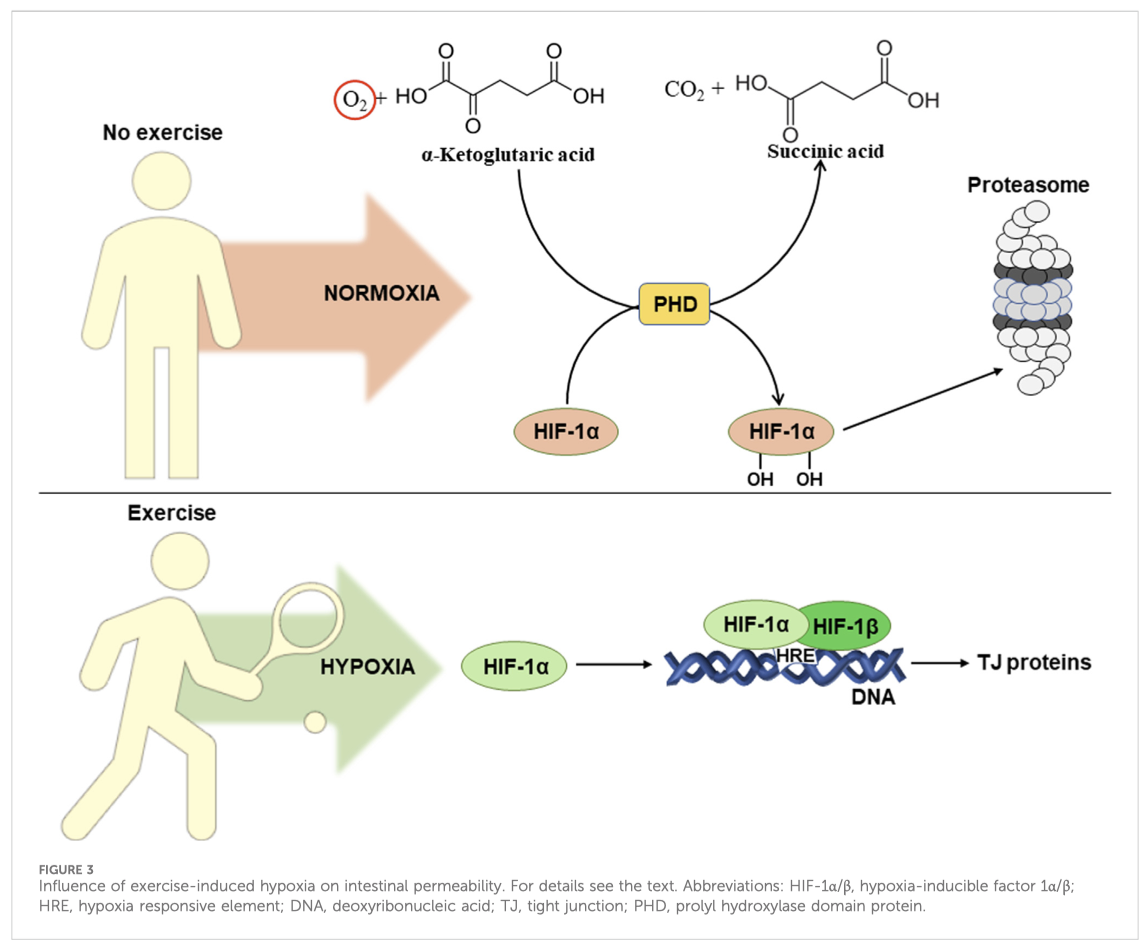
6.2 Exercise-induced hypoxia
It is well known, that exercise causes a redistribution of blood flow between tissues. This leads to the development of hypoxia (decreased oxygen levels) in intestinal epithelial cells and activation of hypoxia-inducible factor alpha (HIF-1α) (Wu et al., 2020). Figure 3 schematically shows the influence of exercise-induced hypoxia on intestinal permeability. In normoxia (normal oxygen levels), prolyl hydroxylase hydroxylates HIF-1α at two proline residues (Pro 402 and Pro 564). This results in ubiquitination followed by subsequent proteasomal degradation of HIF-1α (Lee et al., 2004).
…..omissis
7 Conclusion and perspectives
The intestinal wall is a kind of checkpoint between the external and internal environments of organisms. The wall consists of three layers: mucous, epithelial, and lamina propria. The mucous layer is inhabited by microorganisms, many of which mutually beneficially coexistence within the human body. These microorganisms modulate many if not most living processes: from the development of the immune and nervous systems at early stages of life to the induction of chronic inflammation causing neurodegeneration at aging. Despite the fact that these microorganisms have coexisted with humans for many years, under certain conditions the enteral immune system of the lamina propria can perceive them as foreign and trigger a pro- inflammatory response.
Normally, the intestinal mucosa is semipermeable. It allows selective absorption of nutrients into the bloodstream but prevents the entrance of potentially harmful microorganisms and their waste products from contact with the enteral immune system. An imbalance of the intestinal microbiota, called dysbiosis, can cause a disturbance of intestinal integrity and increase intestinal permeability. Conversely, a healthy composition of the gut microbiota can contribute to the integrity of the intestinal barrier due to increased expression and induction of the assembly of TJ proteins, activation of mucus synthesis, and antioxidant action.
Disruption of intestinal barrier function may trigger development of local and even systemic inflammation.
…..omissis
In general, a vicious cycle of intestinal barrier disruption can be traced here, as excessive intestinal wall permeability provokes the development of chronic low-grade inflammation. The latter is characterized by increased production of pro-inflammatory cytokines and enhanced ROS generation, increasing intestinal barrier dysfunction.
Nutrition looks to be the simplest non-pharmacological effector of integrity and permeability of the intestinal wall. It can have both a negative effect, such as HFD inducing metabolic endotoxemia, or a positive effect, such as a diet rich in plant polyphenols or fermented dairy products, increasing the expression of TJ proteins and promoting the development of beneficial bacteria.
Exercise also can affect gut intestinal permeability. Its effects depend on duration and intensity of exercise. Acute extensive physical exertion often increases intestinal permeability which may be related to the induction of heat stress, that organisms cannot cope with at that time due to insufficient resources. On the other hand, regular low and moderate intensity exercises, that are adaptive in nature, mostly have a positive effect on the integrity of the intestine and decrease its permeability. Potentially, this may be associated with an increase in the steady-state level of HSPs and chronic activation of HIF-1α which activates the transcription of genes responsible for strengthening the intestinal barrier function.
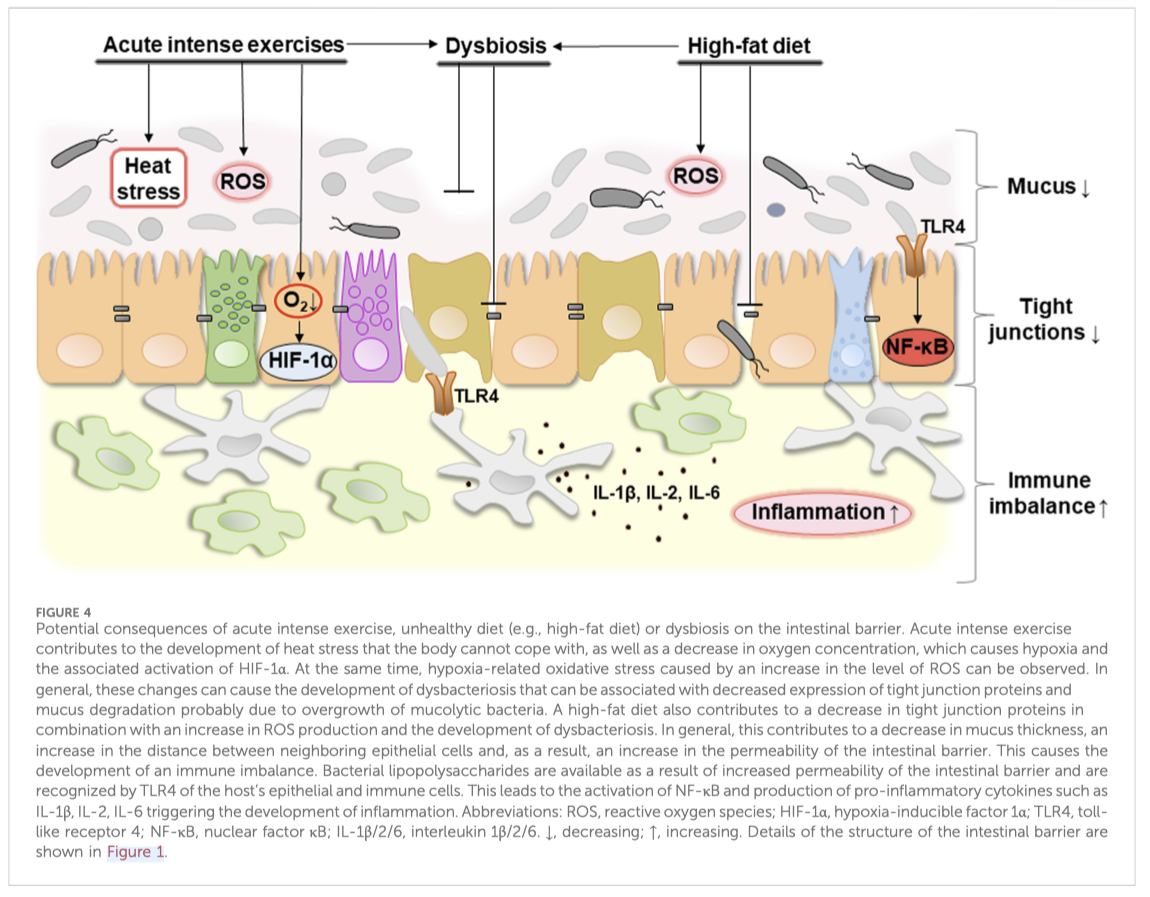
In general, it can be concluded that proper nutrition which promotes a healthy biodiversity of the gut microbiota, combined with moderate exercise, contribute to the integrity of the intestine. Disbalanced nutrition and excessive physical activity can provoke the development of dysbacteriosis and increase intestinal permeability which can potentially lead to a pro-inflammatory response. Figure 4 schematically shows potential consequences of acute intense exercises, unhealthy diet (e.g., high-fat diet), and dysbiosis on the intestinal barrier.
Taking into account all of the above, we can outline the following future prospects:
1. Development of healthy diets to support intestinal homeostasis;
2. Use of fermented dairy products as natural pre-, pro- and postbiotics to promote a healthy gut;
3. Selection of exercises to promote intestinal integrity by frequency, intensity and duration;
4. Study of the role of intestinal HIF-2α during exercise;
5. Systemic investigation of hypoxia-induced oxidative stress as a regulator of intestinal wall permeability.
Most of these perspective avenues are directed to enhance the capability of organisms to cope with disturbing factors. That increases an adaptive capability via preadaptation/hormetic mechanisms. However, some of them may be used “to patch holes” in “leaky” intestinal wall, which is characterized by increased specific permeability of the intestinal epithelium. Intestinal barrier permeability: the infuence of gut microbiota, nutrition, and exercise. Tetiana R. Dmytriv et al. DOI 10.3389/fphys.2024.1380713. PUBLISHED 08 July 2024
Note
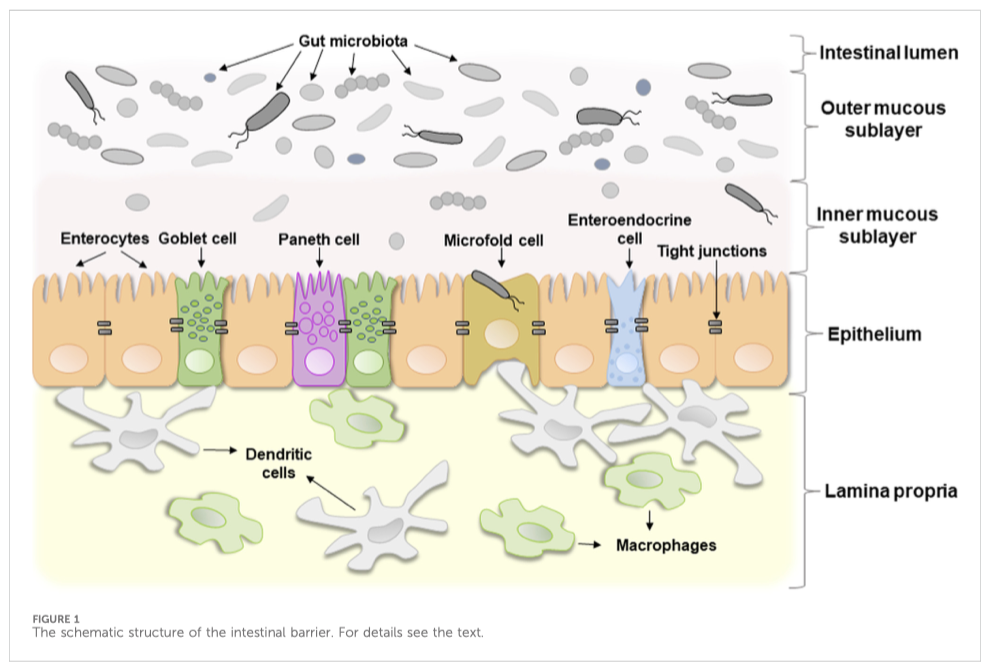
[1] The term “intestinal barrier” emphasizes the barrier function of the intestinal wall which protects organism against invading by bacteria or other microorganisms and potentially toxic components of microorganisms. In fact, it is a complex selective physical barrier that separates the internal environment of the body from the contents of the intestinal lumen (Bischoff et al., 2014). Figure 1 shows a schematic structure of the intestinal barrier. It consists of several layers: i) a mucous layer including inner and outer mucous sublayers inhabited by commensal microorganisms in a different extent, ii) a single layer of epithelial cells, and iii) the lamina propria, which consists of immune cells that instantly react to the invasion of foreign substances (Schoultz and Keita, 2020).
The first layer, the mucous layer, that consists mainly of a mesh polymer called mucin, is located on the side of the intestinal lumen. It is associated with community of commensal microorganisms, including bacteria, fungi, viruses, and parasites, that form the individual microbial community (Chelakkot et al., 2018). A change in the microbial composition that causes a sharp imbalance between beneficial and potentially pathogenic bacteria, including changes in its functional composition, metabolic activity or changes in their local distribution, is called dysbiosis or dysbacteriosis. The latter usually results from loss of beneficial bacteria, overgrowth of potentially pathogenic bacteria, or loss of overall bacterial diversity. This disrupts the homeostatic balance of the intestinal microbiota and has a negative impact on the host’s health. In particular, dysbacteriosis is implicated in a wide range of diseases (DeGruttola et al., 2016).
The second layer, the intestinal epithelium, consists of a single layer of several specialized epithelial cells, such as enterocytes, Goblet cells, Paneth cells, enteroendocrine cells, and microfold cells (Figure 1). Enterocytes form the basis of the intestinal epithelium and play a main role in the absorption of all consumed nutrients. Goblet cells constitute about 10% of specialized epithelial cells. They secrete mucus to protect the intestinal wall from digestive enzymes (Kim and Ho, 2010). Paneth cells contain secretory granules filled with antimicrobial peptides, that are secreted in low amounts constitutively and provide the antimicrobial properties of the intestinal mucosa. Under certain conditions, their secretion can increase dramatically (Yokoi et al., 2019). Enteroendocrine cells produce hormones regulating secretion of digestive enzymes and insulin, peristalsis of the intestine, satiety, and immune response (Bonis et al., 2021). Microfold cells transport bacteria and antigens from the epithelium to enteric immune cells that either activate or suppress the immune response (Jung et al., 2010). All these cell types collectively contribute significantly to gut homeostasis.
The third layer, lamina propria, is located under the epithelium and forms the enteric immune system that consists of a large number of leukocytes with macrophages and dendritic cells being the dominant cell types (Shemtov et al., 2023). Resident intestinal macrophages are located in close proximity to the gut microbiota, with which they often interact. They play a key role in immune sampling of luminal bacteria, contributing to the maintenance of intestinal homeostasis and regulated immune response.
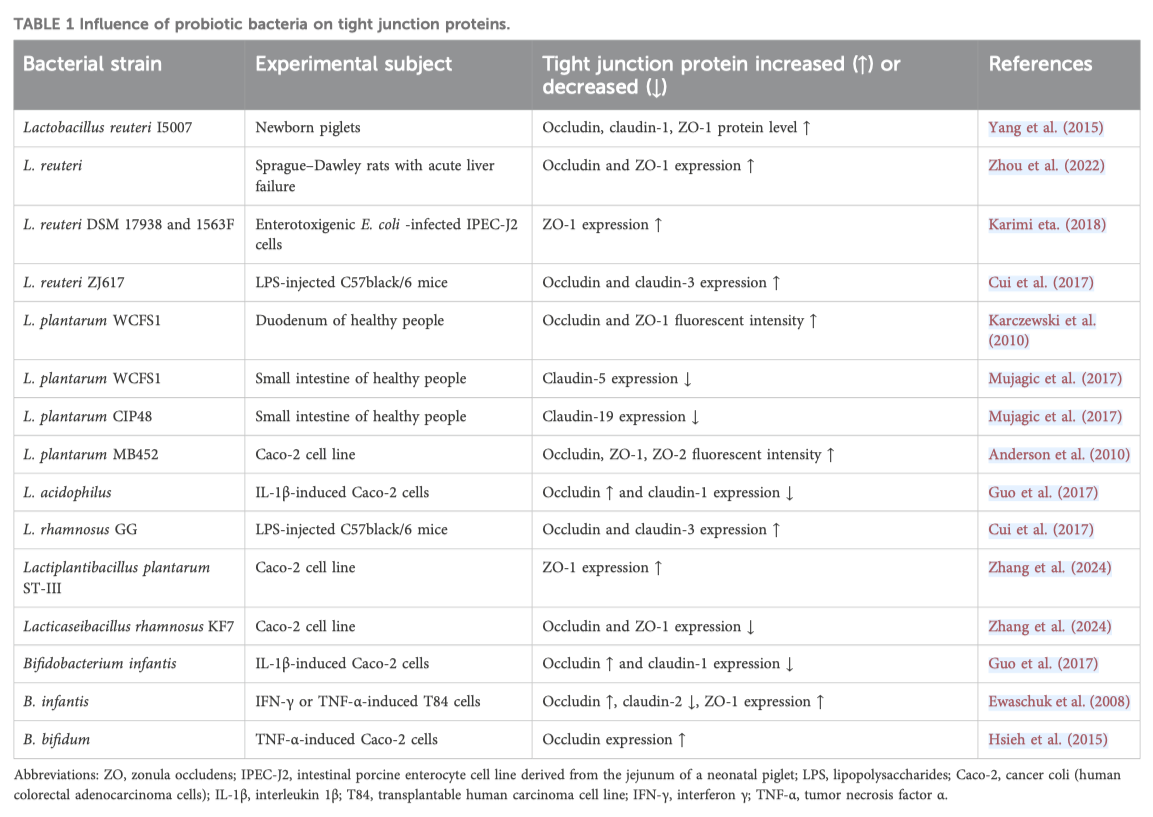
[2] TJ proteins are a complex of transmembrane and cytoplasmic proteins that form tight junctions, which seal cells together to create a selective barrier, maintain cell polarity, and regulate cell processes.
Key words
tight junction, tight junction proteins, inflammation,