La Non-Celiac Gluten Sensitivity (NCGS), aneddoticamente descritta in passato e dal 2010 riconosciuta come una nuova entità clinica, si riferisce a quei pazienti che, senza essere affetti da celiachia né da allergia al frumento IgE mediata [1], presentano una serie di manifestazioni cliniche intestinali ed extraintestinali, che insorgono tempestivamente dopo ingestione di alimenti contenenti glutine e altrettanto rapidamente scompaiono a dieta aglutinata. Nonostante la consapevolezza del dato clinico è una condizione a patogenesi ignota (si ipotizza un ruolo dell’immunità innata) e in cui, in assenza di markers genetici, sierologici e istologi, la diagnosi è largamente ipotetica, posta su base clinica e in base a criteri di esclusione Nei pochi studi presenti in letteratura condotti in cieco vs placebo per verificare la reale risposta all’ingestione di glutine, possono essere sollevate obiezioni metodologiche, i risultati sono controversi, è emerso il ruolo dell’effetto nocebo, e l’effetto glutine- ‐ specifico sembra molto limitato. Da segnalare inoltre recenti evidenze riguardanti molecole, spesso presenti negli stessi alimenti contenenti glutine e in grado di scatenare disturbi sovrapponibili e in comune con la s. dell’intestino irritabile (FODMAPs, fermentable, oligo- ‐, di- ‐ and mono- ‐saccharides and polyols; ATIs, amylase trypsin inhibitors).
Nella NCGS l’esclusione del glutine dalla dieta risolve la sintomatologia entro pochi giorni; nei pazienti che lamentano disturbi dopo ingestione di alimenti contenenti frumento è pertanto necessario escludere sia la celiachia che l’allergia al grano, e quindi confermare la diagnosi attraverso il monitoraggio clinico dopo introduzione di una dieta di esclusione seguita dalla reintroduzione della dieta libera; in assenza di markers specifici e per la verosimile influenza di effetto placebo/nocebo, il percorso diagnostico dovrebbe essere condotto presso le strutture e dalle stesse figure professionali dei Centri di Riferimento e Presidi di Rete per la MC (gastroenterologo dell’adulto o pediatrico, allergo immunologo, specialisti del settore nutrizione).
La prevalenza varia in letteratura dallo 0.6% al 6%, nel 50% dei casi si rileva associazione con gli aplotipi HLA DQ2/DQ8, valore statisticamente non significativo rispetto alla popolazione generale, esiste una netta prevalenza nel sesso femminile e in una variabile percentuale dei pazienti è rilevabile una positività sierologica per gli anticorpi anti gliadina (AGA) di prima generazione, non più utilizzabili per la diagnosi di celiachia per la scarsa accuratezza diagnostica. L’esame istologico della mucosa intestinale risulta nella norma o documenta un aumento dei linfociti intraepiteliali in assenza di atrofia villosa. Sono invece descritti segni di attivazione dell’immunità innata, non glutine- ‐specifica (Tab 4.1).
Quando sospettarla
Nell’età adulta i sintomi possono essere gastrointestinali, assimilabili alla sindrome dell’intestino irritabile oppure reflusso gastroesofageo, nausea, stomatite aftosa [2], epigastralgia [3] (Fig 4.1), associati o meno a sintomi extraintestinali, tra cui prevalgono l’astenia, la confusione mentale, le artralgie e le mialgie, la cefalea, le eruzioni cutanee (Fig.4.2).
Figura 4.1 Sintomi gastrointestinali nella sospetta non-celiac gluten sensitivity (%= percentuale di pazienti)
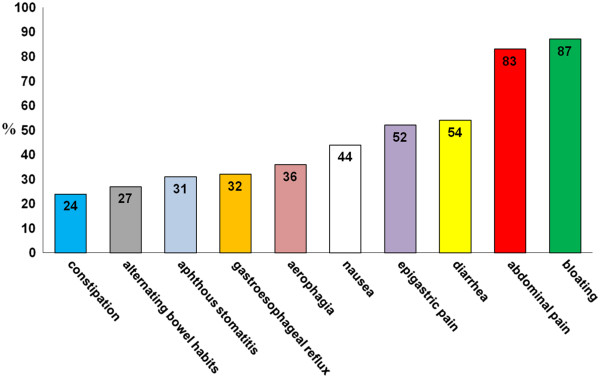
Sintomi gastrointestinali nella sospetta non-celiac gluten sensitivity
Figura 4.2 Sintomi non gastrointestinali nella sospetta non- ‐celiac gluten sensitivity (%= percentuale di pazienti)
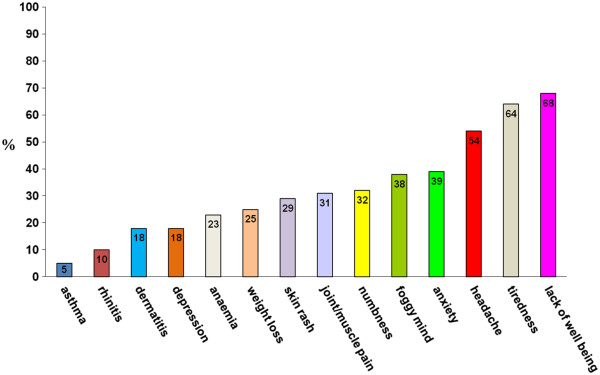
Sintomi non gastrointestinali nella sospetta non- ‐celiac gluten sensitivity
La diagnosi
Al momento la diagnosi di sensibilità al glutine è solo d’esclusione, non esiste un bio-marker specifico né test dedicati, occorre sospettarla quando è possibile dimostrare che la sintomatologia riferita dal paziente è completamente risolta dall’esclusione del glutine e solo del glutine dalla dieta, mentre la sua reintroduzione determina in tempi brevi, ore o giorni, il ripresentarsi dell’intera sintomatologia.
A fronte di questo occorre escludere la MC e l’allergia al grano come già sottolineato.
Nella GS poi non è nota la dose tollerata di glutine né per quanto tempo occorre escludere il glutine dalla dieta: gli studi clinici relativi a questa condizione dovrebbero prevedere l’effettuazione, nelle strutture allergo- ‐immunologiche dedicate, del DBPCT con il glutine; questo test può consentire anche in questa condizione come nell’allergia IgE mediata, un maggior conforto diagnostico e può anche consentire di stabilire quale dose minima può essere tollerata dal singolo individuo.
Note
[1] Allergia alimentare a grano IgE-mediata. I soggetti allergici sviluppano sintomi da minuti a 2 ore dopo l’assunzione di grano. I sintomi includono orticaria, angioe- dema, eritema, prurito, vomito, dolore addominale, tosse, raucedine, wheezing, stridore, distress respiratorio, congestione nasale fino all’anafilassi.
L’allergia IgE-mediata può essere considerata come un’alterazione della risposta immunitaria, ovvero una reazione anormale e specifica verso sostanze, in realtà innocue, percepite come nocive, quindi, attaccate dalle difese immunitarie dell’organismo.
La classe di anticorpi che entra in gioco prende il nome di immunoglobuline E (IgE).
La reazione insorge in seguito a contatto, ingestione o inalazione di sostanze che possono essere di varia natura e che prendono genericamente il nome di allergeni.
La prima esposizione all’allergene determina una sensibilizzazione dell’organismo che produce specifiche immunoglobuline (IgE), senza la comparsa di sintomi. A partire dal secondo contatto, si innescano reazioni a cascata in cui intervengono altri componenti del sistema immunitario, quali i mastociti (a livello tissutale) ed i basofili (a livello ematico).
I mastociti si trovano sotto la superficie cutanea e nelle membrane che rivestono il naso, l’apparato respiratorio, gli occhi e l’intestino. Le IgE, attivate dal legame con l’antigene, vanno a scatenare la risposta allergica legandosi a queste cellule. La degranulazione dei mastociti provoca la liberazione di istamina, leucotrieni e prostaglandine: tali mediatori chimici, agendo su diversi organi e tessuti, provocano l’insorgenza dei sintomi che caratterizzano le risposte allergiche.
È possibile, dunque, identificare tre punti cardine per questa tipologia di reazione:
1. si prevede il coinvolgimento delle IgE;
2. le risposte avvengono rapidamente in seguito al contatto con l’allergene, facilitando il riconoscimento del nesso causa-effetto;
3. lo scatenarsi della risposta è indipendente dalla quantità di allergene con cui l’organismo viene a contatto: si tratta di reazioni NON dose-dipendenti.
[2] (La stomatite aftosa è la comune malattia della bocca, tipica dei giovani e dei giovani adulti, che comporta la ripetuta comparsa di ulcere benigne sulla mucosa orale.)
[3] L’epigastralgia (o dolore alla bocca dello stomaco) è un disturbo molto comune, che si manifesta come un dolore acuto localizzato nella parte superiore dell’addome. Le cause sono molte e legate principalmente a patologie del sistema digestivo.
Bibliografia
• Sapone A, et al. Spectrum of gluten- ‐related disorders: consensus on new
nomenclature and classification. BMC Med 2012;10:13.
• Catassi C, et al. Non- ‐Celiac Gluten sensitivity: the new frontier of gluten
related disorders. Nutrients 2013;5:3839–3853
• Volta U et al. An Italian prospective multicenter survey on patients suspected
of having non- ‐celiac gluten sensitivity. BMC Med. (2014)
Approfondomento:
Esclusione del glutine per i pazienti con NCGS:
Hansen et al. hanno dimostrato che quantità minime di glutine sono sufficienti per influenzare la popolazione del microbiota, abbassando la quantità dei bifidobatteri (batteri buoni) nei pazienti che aderiscono a un regime a basso contenuto di glutine. Leggi di più…..
