Reazioni avverse al grano o a componenti del grano.
La ricerca che presentiamo è un ottimo compendio delle attuali conoscenze sulla sensibilità al glutine non celiaca
“Riassunto: il grano è un alimento base importante a livello globale e fornisce un contributo significativo all’apporto giornaliero di energia, fibre e micronutrienti. Il consumo di più cereali integrali, tra cui il grano, contribuisce a ridurre il rischio del diabete, delle malattie cardiovascolari e del cancro del colon. Tuttavia, i componenti specifici del grano possono anche provocare reazioni fisiche avverse in soggetti sensibili come la celiachia (CD) e l’allergia al grano (WA). Recentemente i media hanno evidenziato una stretta correlazione tra consumo di grano ed effetti negativi sulla salute. Ciò ha motivato molti consumatori a evitare o ridurre il consumo di alimenti che contengono grano / glutine, nonostante l’assenza di CD o WA diagnosticati, sollevando domande sui meccanismi sottostanti e sui possibili effetti di nocebo. Tuttavia, studi recenti hanno dimostrato che alcuni soggetti possono soffrire di reazioni avverse in assenza di CD e WA. Questa condizione è chiamata sensibilità al glutine non celiaca (NCGS) o sensibilità al grano non celiaca (NCWS). Oltre al glutine, il grano e i prodotti derivati contengono molti altri componenti che possono scatenare sintomi, tra cui inibitori dell’α-amilasi e tripsina (ATI), lectine e carboidrati a fermentazione rapida (FODMAP). Inoltre, il modo in cui vengono elaborati gli alimenti, come l’uso di lievito o la fermentazione a lievito naturale, i tempi di fermentazione e le condizioni di cottura, possono influenzare la presenza e la bioattività di questi componenti. La presente recensione descrive sistematicamente le caratteristiche delle intolleranze legate al grano, tra cui l’eziologia, la prevalenza, i componenti responsabili, la diagnosi e le strategie per ridurre le reazioni avverse.
Extract from the study:
Non-Celiac Gluten/Wheat Sensitivity
During recent years a third group of people has been classified who experience symptoms after eating wheat products, but have been diagnosed not to suffer from either WA or CD. Mostly these individuals are self diagnosed wheat intolerant/sensitive. In these individuals, irritable bowel syndrome (IBS)-like gastrointestinal symptoms and extra-intestinal complaints occur, which improve on a gluten-free diet. This group of patients is referred to as “non-celiac gluten sensitivity” (NCGS), or the more recently, “non-celiac wheat sensitivity” (NCWS). Di Sabatino emphasizes that NCWS is not a homogeneous disease syndrome (such as CD and WA), but rather a heterogeneous syndrome (Di Sabatino & Corazza, 2012). It is probable that the underlying causes and mechanisms are not the same for all people with NCWS and that reactions may be caused by different components of wheat or grain (products) and involving different host factors. Ludvigsson et al. (2013) defined NCGS as follows: one or more of a variety of immunological, morphological, or symptomatic manifestations that are precipitated by the ingestion of gluten in individuals in whom CD has been excluded. However, despite the word “gluten” in the currently most cited definition “NCGS,” it is far from certain that the gluten is the (main) cause of the symptoms observed. The more recent term “NCWS” was adopted since it was noted that gluten (NCGS) may not be the real cause (Biesiekierski, Peters, et al., 2013; Skodje et al., 2018). For that reason, we will use the term NCWS as most appropriate in the remainder of this article.
NCWS etiology
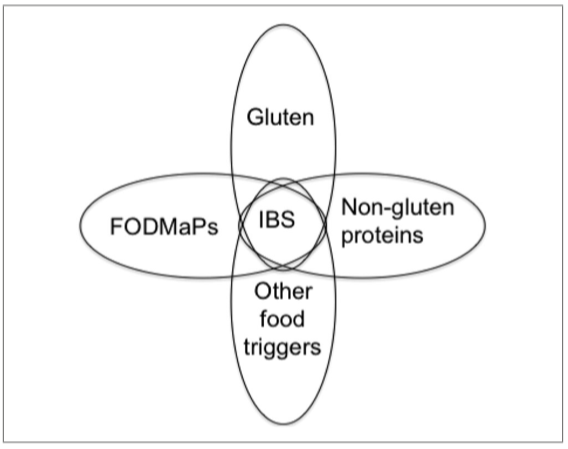
The etiology of NCWS is not completely clear. Intestinal symptoms of NCWS do overlap with CD, but enteropathy (mucosal damage and flattening the brush border) is absent. Serology is generally negative, though increases in anti-tissue transglutaminase (tTG) antibodies, EMA, and DGP antibodies have been reported (Catassi, Elli, et al., 2015). By contrast to CD, NCWS may show signs of an activated innate immune response, whereas, in line with CD, an increased mucosal permeability, may also occur. Cash et al. (2011) found antibodies that could be related to CD (specifically against gliadin) in 7.3% of the IBS patients (total: n = 492) that they examined. However, in only 0.4% was the diagnosis of CD confirmed. Carroccio et al. (2012) observed in a double-blind study that 276 (30%) from 920 diagnosed IBS patients undergoing an elimination diet and a subsequent double blind placebo controlled challenge of these, were suffering from wheat sensitivity as they became asymptomatic (VAS score <10) on the elimination diet and showed symptoms again after the challenge. Two hundred six of the patients (>22.4%) were diagnosed with multiple food hypersensitivity, as they also reacted to the DBPC challenge with cow’s milk protein, egg (n = 120), and/or tomato (n = 112). Only 70 individuals (7.6%) reacted exclusively to wheat (Carroccio et al., 2012). The group that reacted only to wheat demonstrated more association with CD-related biomarkers. For example, 75% of them had the HLA-DQ2/-DQ8 haplotype and 94% of these were found to have increased numbers of lymphocytes in the duodenum. In addition, in one-third of this group of patients intestinal biopsy was positive for EMA, which may indicate a prestage of CD. An overview of different features of CD, WA, and NCWS is given in Table 1 below. For a detailed overview of characteristics and symptoms associated with CD, WA, and NCWS, see Catassi et al. (2017) and Catassi, Elli, et al. (2015).
NCWS prevalence
An international market research agency reported in 2013 that 35% of adult Americans indicated that they are reducing their gluten intake or following a gluten‐free diet because they perceive gluten‐free foods to be healthier (Watson, 2013). Such high numbers are, however, in contrast with data from other large population studies and may vary between countries. In the U.S. National Health and Nutrition Examination Survey 2009–2010 (n = 7,762, CD patients excluded) only 0.55% answered “yes” to the question “are you on a gluten‐free diet?” DiGiacomo et al. (2013) and Rubio‐Tapia et al. (2012) reported similar values: according to their research some 0.63% of North Americans follow a gluten‐free diet. Between 2004 and 2010, 5,896 patients visited the Centre for CD of the Univ. of Maryland (USA) with CD/WA‐like symptoms. Of these patients, 6% met the criteria for NCWS (Sapone et al., 2012). Based on these data, the prevalence in the general population was therefore estimated to be lower than 6%. Two European studies observed a prevalence of respectively 13% self‐reported NCWS in a general population cohort of 1,002 men and women and 6.2% self‐reported NCWS in a general population cohort of 785 adults (Aziz et al., 2014; van Gils et al., 2016). Seventy‐nine percent of these individuals appeared to be females having a significantly increased prevalence of anxiety, depression, chronic fatigue syndrome, and food allergies/intolerances. These observations indicate significant psychiatric comorbidities. In one study, it was noted that hypnotherapy was as effective as a diet low in Fermentable Oligo, Di‐, Monosaccharides, and Polyols (FODMAP) in reducing such symptoms (Peters et al., 2016). It should also be noted that all food allergies, including wheat allergy, are more prevalent in women than in men (Afify & Pali‐Scholl, 2017). The positive predictive value (PPV) of gluten‐related symptoms (defined as the probability that someone with the symptom related to gluten really has NCWS) is very low. For example, Capannolo et al. (2015) studied 392 patients complaining of gluten‐related symptoms. It was found that 26 of these (6.63%) were affected by CD, two (0.51%) by WA, and 27 were diagnosed with NCWS (6.88%). The remaining 337 patients (85.96%) did not experience any change of symptoms with a gluten‐free diet. The authors conclude that self‐perceived gluten‐related symptoms are rarely indicative true gluten sensitivity/NCWS. Due to the current broad consensus definition, it is not yet possible to make a reliable estimate of the number of people suffering from NCWS. It is expected that this will be higher than the number of people with CD, but reliable (more or less substantiated) estimates are scarce and range from 0.5% to 10% of the population (Ludvigsson et al., 2013). The discussion above highlights the complexity of wheat‐related responses and the need for well‐designed studies.
NCWS causing substances
Substances that are often suggested to be involved in NCWS are gluten, non‐gluten proteins, and FODMAPs. Several intervention studies have been carried out that investigated the reactions of people with (alleged) NCWS when put on a diet with and without gluten and/or wheat, in comparison with a control group. Most of the studies were done in groups of patients with IBS, who reported benefits from avoiding wheat. In the studies that showed significant differences, these mainly involved complaints reported after exposure to wheat/gluten in blind challenges studies (the participant in the test did not know whether wheat/gluten or placebo was present in the food). In most studies, no changes were observed in intestinal permeability or in specific (immunological) biomarkers that could explain a potential underlying cause of NCWS. In a few of the studies, CD could not be ruled out because there was no information about the tissue status of the small intestine (Bucci et al., 2013; Jones, McLaughlan, Shorthouse, Workman, & Hunter, 1982; Sapone et al., 2012) whereas in some other studies, patients showed mild intestinal damage which was consistent with the Marsh 1 stage of CD (Figure1). These people may therefore have been erroneously labeled as NCWS patients and would probably be diagnosed with CD after further investigation. On the other hand, such slight damage of the intestinal wall and its associated permeability can also occur after extreme physical exercise, drug and/or excessive alcohol use, as well as are reported in people with IBS. According to various studies, there is also a so‐called “nocebo” effect (a negative expectation effect). For example, a double‐blind placebo‐controlled crossover study by Biesiekierski et al. (2013) showed a significant worsening of overall gastrointestinal symptoms irrespective of the intervention (that is, placebo, low‐gluten, or high‐gluten diet) and interestingly, the symptom scores were highest with the first dietary challenge received. So an order effect was observed, indicating a strong anticipatory symptomatic (that is, nocebo) response, independently of the nature of the dietary challenge (Biesiekierski, Peters, et al., 2013).
It should be noted that a clear relationship of NWCS with gluten alone has not been confirmed by several studies, as they investigated wheat (products) and not gluten per se (Biesiekierski, Muir, & Gibson, 2013; Vazquez‐Roque et al., 2013). In these studies, people were also exposed to other components such as LTPs, ATIs, and rapidly fermentable carbohydrates (that is, FODMAPs), normally also present in wheat. To date, there have been no studies carried out in which wheat‐sensitive people were tested for their reaction to these individual wheat components or combinations of them. Interestingly, studies using tetraploid durum wheat types of older origin were found to reveal less symptoms in IBS patients when compared to modern wheat (Sofi et al., 2014). It may be that differences in genetics, compared to hexaploid breadwheat, and related differences in the composition of these grains play a rol. (Carnevali et al., 2014; Gélinas & McKinnon, 2016; Sofi et al., 2010, 2014).
FODMAPs are defined as Fermentable Oligo, Di‐, Monosaccharides, and Polyols. An increasing number of studies have shown that a low FODMAP diet is associated with symptom improvement in (subgroups of) patients with IBS (Barrett, 2017; Maagaard et al., 2016; Schumann e al., 2018). A number of studies that looked specifically at the effect of a low FODMAP diet in IBS patients reported significant relief of symptoms, mainly abdominal pain and bloating, likely due to less colonic fermentation and associated gas formation (Barrett & Gibson, 2012; Staudacher, Whelan, Irving, & Lomer, 2011; van der Waaij & Stevens, 2014). Studies on long‐term effects are however hardly available. Though, also given the large symptom overlap between IBS and NCWS, wheat‐based products are increasingly being listed as foods that contain fermentable carbohydrates (mainly fructose‐containing polymers called fructo‐oligosaccharides or fructans), being classified as FODMAPs, and may lead to symptoms. However, the quantities of fructans in wheat‐based foods are low and far below the levels that may cause abdominal distress in healthy individuals. For example, the total fructans content in two slices of bread amounts to <0.5 g. In a 35 to 50 g cup of breakfast cereals, this is about 1 g and in pasta about 0.5 g per 150 g portion. Furthermore, it should be noted that FODMAPs will be significantly degraded by the yeast and/or active starter culture during dough fermentation which may lead to complete degradation in sourdough systems (Brouns, Delzenne, & Gibson, 2017). It needs to be noted that there is sound evidence that (long‐term) avoidance of fermentable dietary fibers can impair favorable gut microbiota composition and metabolism, gut function, and health and that eliminating grains from the diet to avoid FODMAPs also results in the elimination of a wide range of other components that are known to be beneficial (Brouns e al., 2017). In this respect a low FODMAP diet is a clinical diet intended for IBS patients that are carefully guided.
NCWS symptoms and diagnosis
Intestinal symptoms commonly reported are as follows: abdominal pain, epi‐gastric pain nausea, gastric acid reflux, episodes of constipation, and diarrhea. Most frequent extra‐intestinal symptoms are poor sleep, tiredness, lack of well‐being, headache, foggy mind, joint/muscle pain, and skin rash/dermatitis. Based on these observations, it is clear that there is significant overlap in symptomatology between NCWS and CD (see also Table 1). At present there is no diagnostic test available for NCWS. Diagnosis is complicated by the fact that people report self‐diagnosed symptoms that overlap with CD and WA. Moreover, there is also a significant overlap between symptoms perceived by individuals suffering from IBS. About 70% of IBS patients consider their symptoms to be food related, with wheat ranking in the top five (Rajilic‐Stojanovic et al., 2015). In 1982, Jones and colleagues reported that of the 21 IBS patients tested, nine reported symptoms after eating wheat without the presence of CD (according to the then applicable diagnostic criteria; Jones e al., 1982). IBS is known to be associated with psychological factors, altered microbiota composition, motility, and/or visceral perception and high placebo response rates (Borghini, Donato, Alvaro, & Picarelli, 2017; Dolan, Chey, & Eswaran, 2018; Ford & Moayyedi, 2010; see Table 1 and Figure 2). Caio, Volta, Tovoli, and De Giorgio (2014) showed that the 93% of the 44 included subjects that suffered from NCWS test positive for anti‐gliadin IgG antibodies and improved significantly following wheat/gluten‐free diets. More recently, Uhde et al. (2016) reported comparable results and showed that reported sensitivity to wheat in the absence of CD was associated with significantly increased levels of soluble CD14 and lipopolysaccharide‐binding protein, as well as antibody reactivity to microbial antigens, indicating systemic immune activation. It should be noted that affected individuals have significantly elevated levels of fatty acid‐binding protein 2, a marker of intestinal damage, and correlates with the markers of systemic immune activation. Furthermore, about 50% of NCWS individuals are positive for first‐generation anti‐gliadin antibodies, mainly IgG, but NCWS is unrelated to the CD genetic markers HLA‐DQ2 and ‐DQ8 (Volta, Caio, Tovoli, & De Giorgio, 2013). The above findings indicate that NCWS is associated with several nonspecific symptoms and factors, complicating an accurate diagnosis (Catassi 2015). At present, suggestions for a possible diagnostic aproach for NCWS have been given by Volta et al. (2013), as follows:
• Gluten ingestion typically elicits the rapid occurrence (in a few hours or days) of intestinal and extra‐intestinal symptoms;
• Symptoms disappear quickly (in a few hours or days) after the elimination of gluten from the diet;
• Reintroduction of gluten causes the rapid recurrence of symptoms;
• Celiac disease must be ruled out by means of negative serology (endomysial and tissue transglutaminase IgA antibodies) and a duodenal biopsy on a gluten‐containing diet;
• Wheat allergy tests (specific IgE as well as skin prick tests), performed on a gluten‐containing diet, must be negative;
• A double‐blind, placebo‐controlled gluten challenge test is needed in each suspected patient to confirm the diagnosis and to exclude a placebo effect induced by gluten exclusion.
Table 1
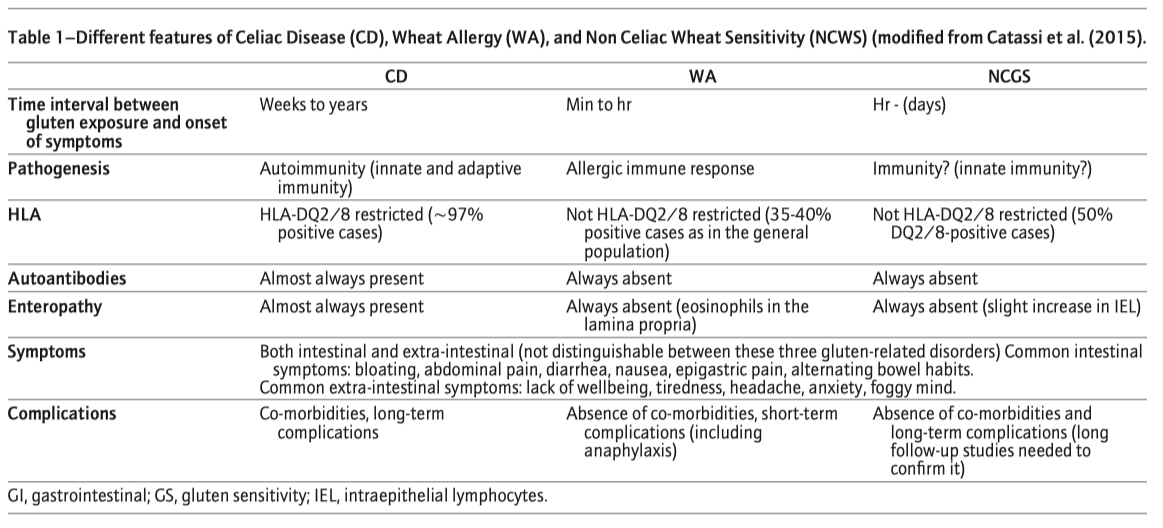
Figure 2: Significant overlap of substances causing symptoms in gluten related disorders and in irritable bowel syndrom (IBS).
One important remark needs to be made here. When stating that symptoms disappear quickly (in a few hours or days) after the elimination of gluten from the diet, one should be aware that gluten avoidance includes avoidance of all other components of wheat (including non‐gluten proteins and FODMAPs).
NCWS threshold and solutions
For many cases of “NCWS” in IBS patients, a GFD and/or wheat‐free diet is advised (Nijeboer, Mulder, & Bouma, 2013). However, there is no reliable information on the threshold of wheat consumption at which people develop complaints. Furthermore, recent studies suggested that FODMAPs and/or probably ATIs, rather than gluten, may be the cause of intestinal distress. The threshold value will depend strongly on the causative component, and the sensitivity of the patient to that component. With regard to dietary fiber, which includes some FODMAPs such as fructans, it is essential to understand that for a healthy intestinal microbiome and intestinal function, the consumption of fermentable carbohydrates is generally recommended. The production of short chain fatty acids and gas during the fermentation of indigestible carbohydrates is not an aspect of disease but merely a normal physiological process in the large intestine, with a range of beneficial effects. Whether the consumption of just 0.5 g to a little more than 1 g of FODMAPs, as present in most breads, can indeed lead to disruptive gas formation is still unclear. As mentioned above, spelt wheat has been reported to have a somewhat lower FODMAP content (1.257 ± 0.213ab g/100 g DM) compared to bread‐wheat (mean value 1.568 ± 0.204 g/110 g DM) (Ziegler & Steiner, 2016). However, it is doubtful that the small differences in the FODMAP contents of different types or cultivars of wheat have any clinical relevance. To what extent this may play a role for some of people with IBS who say they benefit from eating spelt products instead of modern bread wheat products deserves further investigation. Ziegler and Steiner (2016) commented that for wheat bakery products suitable for consumption by IBS patients, the applied long‐lasting dough fermentation method, leading to a potential degradation of 90% of the fructans and raffinose (a trisaccharide that is the second most abundant FODMAP in wheat) in modern and ancient wheat species appears to be substantially more important than the selection of a specific wheat type.
Wheat and Mental‐Psychological Disorders
Apart from the disorders mentioned above, effects on gut–brain axis mediated mental–psychological disorders and well‐being have also been proposed (Hadjivassiliou et al., 2010). Although proposed adverse effects of wheat or gluten on the brain and its functions are beyond the scope of this review on intestinal related adverse reactions and disorders, it should be emphasized that intestinal events can induce effects on the central nervous system by means of the gut–brain axis (Oriach e al., 2016; Vitetta, Vitetta, & Hall, 2018). For this reason, we present some backgrounds here while referring to selected important papers. The term “follow your gut feeling,” when making decisions, appears to have a physiological basis by means of bidirectional communication pathways between gut and brain, in which gut microbiota, the hypothalamic–pituitary–adrenal (HPA) axis, and serotonin metabolism play an important role. There are various lines of growing evidence that the composition of gut microbiota and related metabolism have impacts on brain metabolism and function, including cognition, behavior, mood, and mental disorders (Allen, Dinan, Clarke, & Cryan, 2017; Kennedy, Cryan, Dinan, & Clarke, 2017). Recently, Mu, Yang, and Zhu (2016) describes gut microbiota as a ‘peace keeper’ in brain health. There are some common neurotransmitter and metabolic pathways that play a role in these disorders (Kennedy et l., 2017), which also can be influenced by diet (Julio‐Pieper, Bravo, Aliaga, & Gotteland, 2014). A possible role of gluten and/or wheat has been proposed, based on several single case observations that alleviation of mental disorders can take place after moving to a gluten‐free diet (Lionetti, Leonardi, e al., 2015). It has also been reported that consuming wheat and/or gluten is associated with a variety of psychological symptoms in children, such as depression and attentional problems and that mothers who are unaware of their child having CD more often report depression and anxiety, aggression, and sleep problems (Smith e al., 2017). An important question in this respect is whether gluten, in addition to triggering CD, also the cause of these problems or whether these are simply a comorbidity of CD. Along similar lines, changes in intestinal microbiota composition and activity have been associated with CD, pointing to a possible role of microbiota in its etiology. However, there are at present neither studies demonstrating causality (Galipeau & Verdu, 2014), nor address cause or consequence. For example, autism spectrum disorder is known be associated with a high prevalence of both eating problems and gastrointestinal disorders. In children, eating/feeding problems are complex and multifactorial (Chaidez et al., 2014) and in many cases, it can be expected that the related eating disorders themselves impact on nutrient supply and the gut microbiome, rather than these being the cause of the disorders. Thus, many suggestions that gluten or wheat is a direct cause of mental disorders lack a sound evidence base with respect to components, mechanisms, and cause—effect interactions. In addition, many of the behavioral studies in this field are poorly controlled, have small participant numbers and made use of non‐validated questionnaires (Holingue, Newill, Lee, Pasricha, & Daniele Fallin, 2018; van De Sande, van Buul, & Brouns, 2014). Nevertheless, studies using microbe‐free animal models, using fecal transplantation and/or studies in which antibiotics‐induced microbiota depletion has been carried out, show metabolic and related behavioral changes (Johnson & Foster, 2018). It is thought that such changes are modulated by microbiota‐associated metabolites, which in turn strongly depend on the available substrates that enter the distal part of the small intestine and subsequently the colon, where most microbial metabolism takes place. According to these observations, the modulation of the microbiota by means of ingestion of pro‐ and prebiotics that induce favorable effects on mental functions (psychobiotics) has recently been proposed (Sarkar etal., 2016). Recent work in pigs using distal‐ileal‐antibiotics infusion showed altered neurotransmitter expression occurring simultaneously with changes in both the large intestinal microbiota and the concentration of aromatic amino acids in the colon, blood, and hypothalamus (Gao etal., 2018). According to the researchers, these findings indirectly indicate that intestinal microbiota can affect hypothalamic neurotransmitter expression, which in turn may impact on brain function. These findings point to possible mechanisms and interactions that remain to be shown to play a role in humans. In fact, in humans, so many factors are involved in the day‐to‐day changes in gut microbiota, their metabolism, and gut barrier function, including alcohol, infection, antibiotics, painkillers, stress, and many dietary components (Rybnikova, 2018; Sandhu e al., 2017), that conclusions about gluten or bread as a causal factor for brain disorders cannot be drawn.” (Kelly, Clarke, Cryan, & Dinan, 2016). Clearly there is a need for well‐controlled studies in this respect (Fasano, 2017; Li & Zhou, 2016). Comprehensive Reviews inFood Science and FoodSafety _ Vol.18,2019
Back
