Sindrome dell’intestino irritabile: c’è un ruolo per il glutine?
Uno studio molto importante che evidenzia la sovrapposizione dei sintomi della sindrome dell’intestino irritabile con quelli generati dalla sensibilità al glutine non celiaca dalle ATI e da Fodmaps.
“A tight link exists between dietary factors and irritable bowel syndrome (IBS), one of the most common functional syndromes, characterized by abdominal pain/discomfort, bloating and alternating bowel habits. Amongst the variety of foods potentially evoking “food sensitivity”, gluten and other wheat proteins including amylase trypsin inhibitors represent the culprits that recently have drawn the attention of the scientific community. Therefore, a newly emerging condition termed non-celiac gluten sensitivity (NCGS) or nonceliac wheat sensitivity (NCWS) is now well established in the clinical practice. Notably, patients with NCGS/NCWS have symptoms that mimic those present in IBS. The mechanisms by which gluten or other wheat proteins trigger symptoms are poorly understood and the lack of specific biomarkers hampers diagnosis of this condition. The present review aimed at providing an update to physicians and scientists regarding the following main topics: the experimental and clinical evidence on the role of gluten/wheat in IBS; how to diagnose patients with functional symptoms attributable to gluten/wheat sensitivity; the importance of double-blind placebo controlled cross-over trials as confirmatory assays of gluten/wheat sensitivity; and finally, dietary measures for gluten/wheat sensitive patients. The analysis of current evidence proposes that gluten/wheat sensitivity can indeed represent a subset of the broad spectrum of patients with a clinical presentation of IBS. (J Neurogastroenterol Motil 2016;22:547-557). Umberto Volta, Maria Ines Pinto-Sanchez et al.
Extrac from the study:
…..omissis. Experimental Evidence for a Role of Wheat Components in Irritable Bowel Syndrome. Different mechanisms have been proposed to explain how gluten may trigger gastrointestinal symptoms in the absence of celiac disease (Figure).
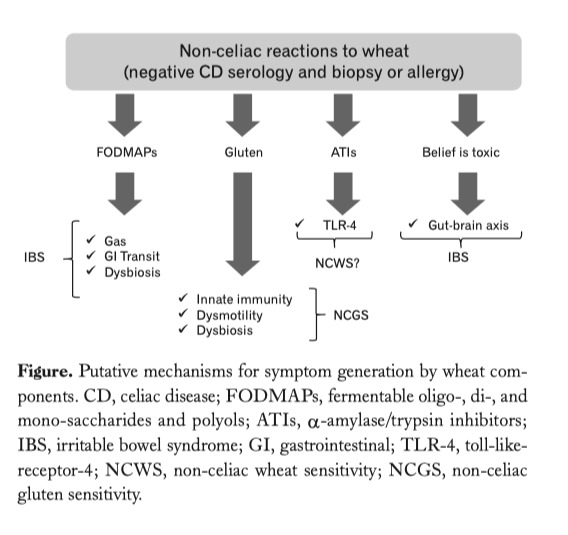 In vitro studies have demonstrated that digests of gliadin increase the expression of co-stimulatory molecules and the production of proinflammatory cytokines in monocytes and dendritic cells (40,57,58). Certain “toxic” (that only stimulates the innate immune response) gliadin-derived peptides such as the 31-43mer, may evoke epithelial cell dysfunction, increased IL-15 production and enterocyte apoptosis (59). Recent studies have demonstrated increased expression of TLR-2 in the intestinal mucosa of non-celiac compared to celiac patients, suggesting a role of the innate immune system in the pathogenesis of non-celiac reactions to gluten or other wheat components (49). Other studies have shown that monocytes from HLA-DQ2+ non-celiac individuals spontaneously release 2-3 fold more IL-8 than monocytes from HLA-DQ2 negative patients. This suggests that patients without celiac disease (no enteropathy and negative specific serology), but with positive HLA-DQ2 status, may represent a subpopulation reacting mildly to gluten (60). In terms of gut dysfunction, gluten sensitization in mice has been shown to induce acetylcholine release, one of the main excitatory neurotransmitters in the gut, from the myenteric plexus (57).
In vitro studies have demonstrated that digests of gliadin increase the expression of co-stimulatory molecules and the production of proinflammatory cytokines in monocytes and dendritic cells (40,57,58). Certain “toxic” (that only stimulates the innate immune response) gliadin-derived peptides such as the 31-43mer, may evoke epithelial cell dysfunction, increased IL-15 production and enterocyte apoptosis (59). Recent studies have demonstrated increased expression of TLR-2 in the intestinal mucosa of non-celiac compared to celiac patients, suggesting a role of the innate immune system in the pathogenesis of non-celiac reactions to gluten or other wheat components (49). Other studies have shown that monocytes from HLA-DQ2+ non-celiac individuals spontaneously release 2-3 fold more IL-8 than monocytes from HLA-DQ2 negative patients. This suggests that patients without celiac disease (no enteropathy and negative specific serology), but with positive HLA-DQ2 status, may represent a subpopulation reacting mildly to gluten (60). In terms of gut dysfunction, gluten sensitization in mice has been shown to induce acetylcholine release, one of the main excitatory neurotransmitters in the gut, from the myenteric plexus (57).
This correlates with increased smooth muscle contractility and a hypersecretory status with increased ion transport and water movements (57). These functional effects induced by gluten were not accompanied by mucosal atrophy, and were not observed after sensitization with non-gluten proteins. Interestingly gluten-induced gut dysfunction was particularly notable in mice transgenic for the human celiac gene HLA-DQ8 (57).
ATIs, a group of wheat proteins that confer resistance of the grain to pests, are strong inducers of innate immune responses via TLR4 and via the myeloid differentiation factor 88-dependent and -independent pathway (40). This activation occurs both in vitro and in vivo after oral ingestion of purified ATIs or gluten, while gluten-free cereals display no or minimal activities (61). The role of ATIs in IBS is not yet known, however there is clear description of a mechanism that could be involved in the generation of gut dysfunction and symptoms. These mechanisms are different from those proposed for gluten and thus it is conceivable that they could co-exist in given patients or have a synergistic effect.
Clinical Evidence for a Role of Wheat Components in Irritable Bowel Syndrome. IBS and celiac disease are common conditions, and they may overlap by chance. However, celiac disease is 3-4 times more common in IBS patients compared to controls suggesting that both disorders could be mechanistically associated. Active screening by celiac serology and biopsy reveals that 4% of patients labeled as IBS have underlying celiac disease (62-65). This overlap between the 2 conditions is different from the issue of wheat components, including gluten, causing functional symptoms in patients without celiac disease (39). Clinical studies have revealed that a subgroup of IBS patients in whom celiac disease and wheat allergy were ruled out, displayed intestinal and extra-intestinal symptoms after wheat ingestion. Three consensus conferences defined this new syndrome as nonceliac gluten sensitivity (NCGS) or, alternatively, non-celiac wheat sensitivity (NCWS) given the possibility for immune reactions or intolerances to other wheat components as explained above (66-68). It is important to bear in mind the complexity of food hypersensitivities and intolerances, many of which could coexist in the same patient. This constitutes a confounder that we must be aware of for the design and interpretation of clinical trials (69).
The prevalence of NCGS/NCWS is still not clearly established mainly due to the hitch in standardizing international diagnostic criteria. In United States this condition seems to be identified more frequently in tertiary referral centers than in primary care. Data obtained in primary care practice, from the National Health and Nutrition Examination Survey (NHANES), indicate that the estimated prevalence of NCGS/NCWS was 0.6% over 7762 subjects (70). On the other hand, data published from the Celiac Disease Center in Baltimore (University of Maryland), showed a prevalence of suspected NCGS/NCWS of 6% over 5896 subjects (66). Moreover, an Italian multicenter study prospectively evaluated the prevalence of NCGS/NCWS and celiac disease in pediatric and adult centers for gluten related disorders (71). Among 12 255 subjects consecutively investigated during a one-year survey, 391 (3.2%) cases of suspected NCGS/NCWS and 340 (2.8%) celiac patients were identified. Based on these results, it is possible to estimate a ratio between NCGS/NCWS and celiac disease of 1.15:1, data in line with the NHANES survey. Although NCGS/NCWS can occur at any age, this condition seems to occur more frequently in adulthood with a mean age at diagnosis of 40 years. The female gender, similarly to IBS, is more affected by this sensitivity with a ratio up to 5:1.71. Some clinical trials have attempted to investigate underlying functional and immune abnormalities in patients with a clinical picture of NCGS/NCWS. Initial results showed that there is increased expression of TLR2 in the small intestine of patients with NCGS/ NCWS (49). This was in contrast with the normal expression of IL17A, IL-6, IFN- g , IL-17, and IL-21 in the duodenal mucosa of these patients, which seemed to rule out the contribution of adaptive immunity (72). Recent work has suggested increased IFN- g levels in small intestinal biopsies of NCGS/NCWS patients following a short-term gluten challenge (73). Although this cytokine is a key mediator of T-helper 1 adaptive immune responses, it could also be produced by innate cells such as intraepithelial lymphocytes (IELs) (73).
Another relevant pathogenic aspect that has been investigated in NCGS/NCWS pertains to possible functional changes, such as the increase of intestinal permeability. Using the lactulose/mannitol test, IBS patients with self-reported sensitivity to gluten did not exhibit significant alterations in sugar permeability. This may be related to the population involved or technical difficulties in the interpretation of clinical permeability tests. However, in contrast to this study, Vazquez-Roque et al, (52) using the same method, found increased intestinal permeability in a subgroup of HLA-DQ2/DQ8+ NCGS/ NCWS patients with IBS-D, once again raising the possibility of a particularly vulnerable subpopulation. This underscores the importance of careful patient phenotyping in studies involving functional symptoms and adverse reactions to food components. It also raises the concept of a subgroup of genetically predisposed individuals carrying celiac markers, but without active celiac disease, that may be more sensitive to low-grade inflammation or functional changes induced by gluten. This will need to be confirmed in large clinical trials.
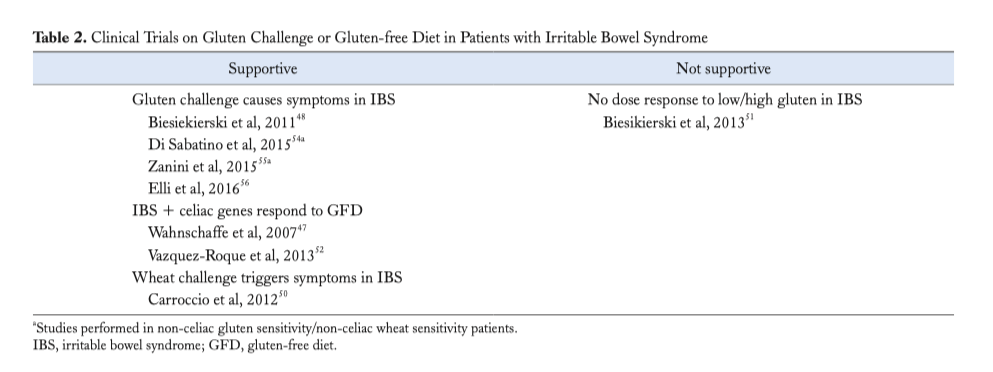
An initial study enrolling IBS patients fulfilling criteria for NCGS/NCWS showed symptom recurrence following gluten reintroduction (48). In a second trial from the same group, (51) IBS patients that responded to a gluten-free diet were challenged with low dose of gluten (2 g/day), high dose of gluten (16 g/day) or whey protein (16 g/day) after 2 weeks of low dose FODMAPs diet. After the challenge, gluten evoked neurological, but not intestinal, symptoms. It is worthwhile to note that patients increased their symptoms with all challenges, including placebo, and that gluten challenge did not show a dose response. Carroccio et al (50) demonstrated that IBS patients randomized to receive whole wheat vs placebo, had a significant worsening of their intestinal and extra-intestinal symptoms after wheat ingestion. A recent DBPCC trial used pure gluten vs rice starch (control)-containing capsules to clarify the exact role played by gluten in symptom generation in patients with highly suspected NCGS/NCWS (54). The results showed that pure gluten ingestion induced recurrence of a variety of symptoms including, bloating, abdominal pain, foggy mind, aphthous stomatitis, headache and depression, but not in all of NCGS patients. Two recent DBPCC trials (55,56) performed in IBS patients self-diagnosed as “gluten/wheat sensitive” strongly suggested that gluten/wheat was responsible for symptom generation in up to one-third of IBS patients. Overall the results indicated that gluten and other wheat proteins cause symptoms in the absence of celiac disease in a subset of the IBS population (Table 2).
The dilemma is that a gluten-free diet may also be low in FODMAPs. Some studies have assessed the efficacy of lowFODMAPs diet in IBS with variable results (Table 3). However, none of these trials included a placebo group, thus introducing a possible bias hampering the actual value of a low-FODMAPs diet both in terms of diagnosis and as a therapeutic intervention. A recent DBPC trial (without crossover) comparing a low-FODMAP diet with traditional dietary advice in IBS, showed no difference among the 2 strategies, thus renewing controversy of the specificity of therapeutic effect of FODMAPs exclusion in the general IBS population (74).
…..omissis. Can We Diagnose Non-celiac Reactions to Wheat/Gluten? NCGS/NCWS is often considered a self-diagnosis, usually reported by the patient. For the physician, it is based on the thorough evaluation of the clinical features according to the indications proposed by the Consensus Conferences on this syndrome (66-68). Identification of biomarkers that allow us to diagnose NCGS/NCWS with more specificity and differentiate it from the unselected IBS population will be key to define and manage this condition (78,79).
The clinical picture of patients with NCGS/NCWS is characterized by symptoms occurring shortly after consumption of wheat/ gluten containing meals and disappearing or recurring in a few hours/days after specific withdrawal or challenge (66-68). Symptoms that characterize IBS, such as bloating, abdominal discomfort/pain, altered bowel habits and tiredness, are present in NCGS/NCWS. It has been suggested that a clinical distinction can be made between general IBS and NCGS/NCWS because of more extra-intestinal manifestations involving the central and/or peripheral nervous system, joint/muscle (“fibromyalgia-like”) and skin manifestations in the latter. Due to the lack of biomarkers, the diagnosis of NCGS/ NCWS remains highly presumptive being based only on clinical and exclusion criteria (80,81). The improvement of symptoms after a gluten-free diet, which is regarded as the major diagnostic criteria for NCGS/NCWS, might be due to a placebo effect which often follows the elimination of some foods from the diet. In this context it is important to stress the negative potential influence (“nocebo” effect) generated by media on the “deleterious effect of wheat consumption”. There is no scientific evidence to support that gluten/wheat consumption is deleterious to the overall population and such sensitivity seems to be limited to a subpopulation of patients that present with IBS symptoms. Despite some disproportionate press on one hand, and some healthy skepticism on the other, there is no doubt that awareness and interest in NCGS/NCWS continues to grow. A specific diagnosis requires the development of a biomarker. Antibodies to native gliadin (AGA) have been suggested as a potential diagnostic marker for NCGS/NCWS diagnosis, in the absence of specific celiac serology such as tissue transglutaminase, endomysial and deamidated gliadin antibodies (84). AGA have been detected in the sera of about half of NCGS/NCWS patients being predominantly of the IgG class. Although AGA are not specific for NCGS/NCWS being detectable in various conditions, ie, autoimmune disorders, connective tissue diseases and even in healthy controls, their positivity, especially at high titers, in patients with a clinical picture suggestive of gluten/wheat sensitivity may be regarded as a diagnostic adjunct. In parallel to symptom resolution, AGA normalized in almost all patients with NCGS/NCWS within 6 months of gluten-free diet (85).
In cases with suspected NCGS/NCWS, the most important clinical issue is to rule out celiac disease while the patient is on a gluten-containing diet. Patients with NCGS/NCWS have normal duodenal mucosal histology, although an increased number of IELs ranging from 25 to 40/100 epithelial cells are found in at least 40% of cases, suggesting an accompanying low-grade inflammation (71). There is no increase of T-cell receptor g / d IELs in biopsies of patients with NCGS/NCWS. Data on HLA complex and NCGS/ NCWS are unclear. Some studies found that HLA-DQ2 and/or HLA-DQ8 genes were present in ~50% of NCGS/NCWS patients, which is slightly higher than in the general population (84). On the other hand, some studies have suggested that IBS patients who carry celiac susceptibility genes are more likely to respond to the gluten-free diet (52). This apparent controversy may be explained by the fact that overall the NCGS/NCWS population may be heterogeneous and responsive to multiple stimuli. It could be speculated that those with celiac susceptibility genes may be more prone to develop mild immune responses and gut dysfunction to gluten. Up to 20% of NCGS/NCWS show mild laboratory abnormalities, such as low levels of ferritin, folic acid, vitamin D and B12, most likely related to a minimal inflammatory state in the intestinal mucosa. Evidence of osteopenia detected by bone densitometry has been found in about 50% of NCGS/NCWS (87). Finally, recent data reported a high prevalence of serum autoantibodies (antinuclear antibodies, ANA) and a frequent association with autoimmune disorders (ie, Hashimoto’s thyroiditis) in patients with NCGS/NCWS (88,89).
…..omissis. Conclusions. Foods can be triggers of gastrointestinal and extra-intestinal symptoms in a proportion of IBS patients. Inside the spectrum of the so-called food hypersensitivity, NCGS/NCWS has been recognized as a newly identified gluten-related disorder which presents clinically with overlapping symptoms of IBS (66-689). Along with gluten, other wheat and food components, have emerged as functional digestive symptom triggers. Within wheat, a potential culprit of symptom generation is ATI, a still poorly investigated soluble protein fraction of wheat. A role for FODMAPs, detectable not only in gluten-containing cereals (wheat, rye, and barley), but also in milk, honey, and legumes, has been proposed, although a recent DBPC trial did not find this restriction more efficient than traditional dietary advice for IBS patients. We have discussed the evidence for some specific dietary components, such as gluten and other wheat components, to cause functional digestive symptoms and extra-intestinal manifestations matching the current criteria for IBS. As with overall IBS, the mechanisms underlying symptom generation in the subgroup of patients with NCGS/NCWS are still poorly understood. Our review focused on clinical features with the intent to expand current knowledge in this area of gastroenterology. A better understanding of food hypersensitivity and intolerances as well as the development of biomarkers will enable physicians to design tailored dietary approaches to treat patients with food-related functional bowel disorders.
Back
