Gluten: amino acids, digestion, toxic peptides
Gliadin and Glutenin
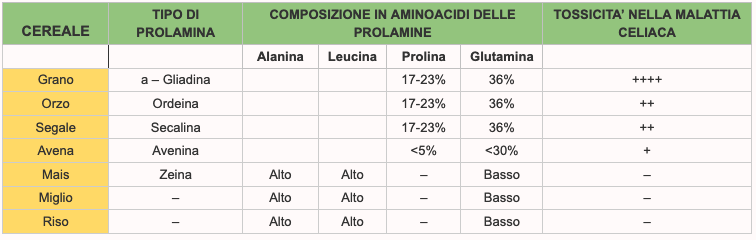
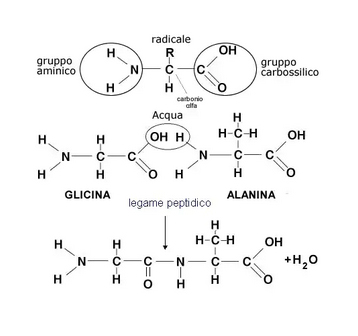
They are the wheat proteins (gliadin, soluble in alcohol and glutenin, insoluble in alcohol) and are composed of amino acid chains (1). Gliadin is made up of the union of about 100-200 amino acids (the main cause of celiac disease), and glutenin, consisting of a combination of about 2,000-20,000 amino acids. The covalent bond that unites two amino acids also takes the name in biochemistry of “peptide bond”. A chain of multiple amino acids linked through peptide bonds takes the generic name of peptide or polypeptide or oligopeptide if the number of amino acids involved is limited; one or more polypeptides, sometimes accompanied by other auxiliary structures or ions called cofactors or prosthetic groups, constitute a protein. amino-acids (or amminoacids) are the primary structural unit of proteins. We can therefore imagine the amino acids as bricks that, united by a glue called peptide bond, form a long sequence that gives rise to a protein. Alcohol soluble cereal proteins are called: prolamines.
The wheat prolamine is gliadin, that of barley is hordein; that of rye is secaline, that of avena is avenin. The different types of prolamins contain different amino-acids and the higher the content of proline and glutamine (which are some of the amino-acids that compose it) the more the prolamine, and therefore the peptides of that cereal will be toxic (2) for the affected patient from celiac disease. The highest levels of proline and glutamine are in wheat, barley and rye. Also glutenins have some toxic sequences for celiacs but they appear to be much less active in soliciting the adverse response of the humanitarian system of man.
Digestion of gliadin and glutenin
Within the stomach and duodenum the bonds that combine amino acids and oligopeptides are digested through the action of dedicated enzymes. Digesting means reducing the oligopeptides that make up the two proteins (gliadin and glutenin) into the smallest possible fragments. The small intestine is in fact able to absorb only the single amino acids or fractions with very few amino acids.
The digestion of gliadin and glutenin is linked to the length of the polypeptides that compose them, to the strength of the bonds existing between the amino acids and among the polypeptides, to the sequence / nature of the single amino acids.
Proteins are molecules resistant to digestion, so we must use many enzymes to reduce them into smaller and smaller pieces. Protein digestion begins in the stomach, where hydrochloric acid creates the environment suitable for the enzyme pepsin that makes the first cuts. But the bulk of the work begins later in the intestine. The pancreas produces many proteolytic enzymes, the most important of which is trypsin, which reduces protein chains in fragments composed of a reduced number of amino acids. Then, other enzymes, on the surface of the intestinal cells and inside the cells, operate further reduction in very small fragments or / and single amino-acids that are absorbed starting from the duodenum throughout the jejunum and the ileum (the three sections of the small intestine) through the intestinal villi to be, then, assimilated for the synthesis of new proteins and not only. After being absorbed they will reach the liver where they can:
• be used as such to perform particular functions (involved in the immune response, in the synthesis of hormones and vitamins, in the transmission of nerve impulses, in the production of energy and as catalysts in many metabolic processes)
• participate in protein synthesis, a reverse process to the digestive process that aims to provide the body with materials for the growth, maintenance and reconstruction of cellular structures
• if present in excess they are used for energy purposes (gluconeogenesis) or converted into fat storage.
Partial digestion of gliadin and glutenin
Gliadin and glutenin are not assimilable as they are (the peptides that compose them are composed of many amino-acids that form long chains) from the intestine; the hydrolysis (fragmentation) of peptides in single amino-acids or very small fragments (less than 9 amino-acids) is, therefore, fundamental for being able to cross the intestinal epithelium; the larger fractions, in general, are eliminated with feces. Another factor that affects the digestibility of these proteins is the type of constituent amino-acids: the high content of proline and glutamine makes these proteins resistant to complete digestion in the small intestine. “Prolamins (gliadins and glutenins) have a high content of proline (15%) and glutamine (35%) and, depending on the cereal, they have been termed secalin for rye, hordein for barley, avenin for oats, and gliadin for wheat. The high concentration of these amino acids, especially proline, limits proteolysis by gastrointestinal enzymes, preventing the complete degradation by human gastric and pancreatic enzymes. Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and
Product Quality . Nina G. Heredia-Sandoval , Maribel Y. Valencia-Tapia , Ana M. Calderón de la Barca and Alma R. Islas-Rubio . Received: 1 May 2016; Accepted: 27 August 2016; Published: 30 August 2016.”
Tolerability of gliadin and glutenin
In some people some specific fragments coming mainly from α-gliadins and secondarily from HMW-GS trigger celiac disease (Gilissen et al., 2014). These fragments are peptides consisting of a sequence of nine amino acids that come from proteins rich in proline and glutamine (prolamine), which are resistant to digestion (Bethune and Khosla, 2008). These fractions are generally also the most resistant to gastro-intestinal digestion. Therefore, it has been hypothesized that gliadins, although difficult to hydrolyse from gastro-enteric enzymes, remain immunologically inactive in most people.
“Alimentary protein digestion followed by amino acid and peptide absorption in the small intestinal epithelium is considered an efficient process. Nevertheless, unabsorbed dietary proteins enter the human large intestine as a complex mixture of protein and peptides.53,63 The incomplete assimilation of some dietary proteins in the small intestine has been previously demonstrated, even with proteins that are known to be easily digested (e.g., egg protein).64,65 The high proline content of wheat gluten and related proteins renders these proteins resistant to complete digestion in the small intestine. As a result, many high molecular weight gluten oligopeptides arrive in the lower gastrointestinal tract.66 While gluten peptides pass through the large intestine, proteolytic bacteria could participate in the hydrolysis of these peptides. 81Gluten Metabolism in Humans. Alberto Caminero, … Javier Casqueiro, in Wheat and Rice in Disease Prevention and Health, 2014”
The case of the peptide 33 mer
All grains / farri contain fractions (toxic) that activate the reaction of the human immune system in some individuals causing celiac disease (estimates: 1-2% of the population) in others non-celiac gluten intolerance (estimates: 5 -10% of the population). The quantity and quality of these fractions is very varied and should therefore be estimated for each variety. Among all the fractions toxic – as mentioned above – one in particular has been identified as the most important: the 33 mer of the α-gliadin.
“The α-gliadin 33-mer is one of the digestion-resistant gluten peptides that is highly reactive to isolated celiac T cells and is the main immunodominant toxic peptide in celiac patients. It is located in the N-terminal repetitive region of α-gliadin and contains six overlapping copies of three different DQ2-restricted epitopes (Figure 6) [86]. Using RNA-amplicon sequencing (NGS) technology it was shown that α-gliadins can be separated into six types and only one type contains all the immunogenic peptides and epitopes, whereas the other five types do not contain all the epitopes disabling 33-mer peptide formation [30]. Thus, distinct types of α-gliadins differ mainly in the number of repeat blocks consisting in interspersed motifs PFPPQQ and PYPQPQ. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxi=ication Capabilities. Anastasia V. Balakireva and Andrey A. Zamyatnin Jr. Nutrients: Received: 28 August 2016; Accepted: 11 October 2016; Published: 18 October 2016.”
The 33 mer peptide is also present in all soft wheat and spelt flours with different levels (from 91-603 μg / g flour). It should be emphasized that in the monococcus, dicocco and durum wheat the 33 mer is absent as it is encoded in the DD genome not present in the cited grains.
“Coeliac disease (CD) is triggered by the ingestion of gluten proteins from wheat, rye, and barley. The 33-mer peptide from α2-gliadin has frequently been described as the most important CD-immunogenic sequence within gluten. However, from more than 890 published amino acid sequences of α-gliadins, only 19 sequences contain the 33-mer. In order to make a precise assessment of the importance of the 33-mer, it is necessary to elucidate which wheat species and cultivars contain the peptide and at which concentrations. This paper presents the development of a stable isotope dilution assay followed by liquid chromatography tandem mass spectrometry to quantitate the 33-mer in `lours of 23 hexaploid modern and 15 old common (bread) wheat as well as two spelt cultivars. All =lours contained the 33-mer peptide at levels ranging from 91–603 μg/g =lour. In contrast, the 33- mer was absent (<limit of detection) from tetra and diploid species (durum wheat, emmer, einkorn), most likely because of the absence of the D-genome, which encodes α2-gliadins. Due to the presence of the 33-mer in all common wheat and spelt flours analysed here, the special focus in the literature on this most immunodominant peptide seems to be justified……Omissis…..
“Quantitation of the immunodominant 33-mer pep&de from α-gliadin in wheat flours by liquid chromatography tandem mass spectrometry.
Kathrin Schalk , Christina Lang , Herbert Wieser , Peter Koehler & Katharina Anne Scherf. Scienti’ic Reports volume 7, Article number: 45092 (2017).”
The case of the 13mer petide
The 13-mer LGQQQPFPPQQPY peptide located at position 31-43 of an α-gliadin known as A-gliadin (Kasar-da et al., 1984) is very active in inducing lesions of the intestinal mucosa and apoptosis of the enterocyte (Maiuri et al., 2003). This peptide, or its variant PGQQQPFPPQQPY in which the residue L (leucine) at position 31 is replaced by P (proline), is present in many currently known a-gliadin sequences (Kasarda et al., 1984; Kasarda and D’Ovidio, 1999; Arentz-Hansen et al., 2000). It does not show an immunogenic activity on T cells, but possesses the ability of prolamines to agglutinate undifferentiated K562 cells, hinders the healing of patients with atrophic duodenal mucosa and activates the mechanisms of innate immunity in the mucosa of gluten-free celiac patients (De Ritis et al., 1988; Gianfrani et al., 2005).
(1) – Aminoacidi presenti nella gliadina e glutenina
(2) Translational research
Identified the repertoire of toxic gluten peptides. Therapeutic applications for celiac disease. Carmen Gianfrani comments on Radio3 Scienza the recent study published in Science Traslational Medicine. This study, coordinated by Dr. Bob Anderson of the Walter and Eliza Hall Institute of Medical Research in Melbourne, in collaboration with the Universities of London, Oxford and the CNR of Avellino, identified the gluten sequences responsible for immunological toxicity in the great majority of analyzed celiac patients. Through an innovative method, which uses a short oral load of foods containing gluten and the analysis of immune response in peripheral blood, about 3,000 different peptides were analyzed in a cohort of 226 adult celiac patients. The most important fact is that, whether the patient eats wheat or rye or barley, only 3 are the peptide sequences responsible for the inflammatory response. The traslational application of this study is represented by the formulation of a therapeutic vaccine, currently in phase 1b of the clinical trial, which is based on repeated subcutaneous injections of a single peptide that includes the 3 toxic sequences, in order to induce the celiac disease immunological tolerance to gluten-containing food. (CNR 30 July 2010)
Depeening:
Classification of amino-acids. Only twenty of the various naturally occurring amino-acids (currently over five hundred) are involved in protein synthesis. From the nutritional point of view, these amino-acids can in turn be divided into two large groups: that of essential amino-acids and that of non-essential amino-acids. amino-acids are defined as essential that the human body cannot synthesize in sufficient quantity to meet its own needs. There are eight for the adult and more precisely: phenylalanine, isoleucine, lysine, leucine, methionine, threonine, trypan and valine. During the period of growth to the eight mentioned, a ninth must be added, histidine, in consideration of the fact that in this period the requests for this aminoacid are higher than the synthesis capacity.
The primary function of amino-acids is to intervene in protein synthesis, necessary to cope with the body’s cellular renewal processes. In addition to this function, called “plastic”, amino-acids also have a modest but not negligible importance in energy production (branched amino acids).
NOTE
1 – From the chemical point of view the aminoacid is an organic compound containing a carboxylic group (COOH) and an aminogroup (NH2). In addition to these two groups, each aminoacid is distinguished from the others by the presence of a residue (R) also known as the side chain of the aminoacid.
2 – Cysteine and tyrosine are considered as semi-essential amino-acids, as the body can synthesize them starting from methionine and phenylalanine. These are conditionally essential amino-acids (arginine, glycine, glutamine, proline and taurine) those amino-acids that play a fundamental role in maintaining homeostasis and body functions in certain physiological situations. In some pathological conditions these amino-acids may not be synthesized at sufficient speed to meet the real needs of the body. Arginine is assuming considerable importance, as a precursor of nitric oxide, for the many functions that the latter performs in cellular activity, in the transduction of biological signals and in immune defense
3 – CONTENT IN ESSENTIAL amino-acids: those proteins containing all the essential AA in quantity and in balanced relationships can be defined complete. In general, animal proteins are complete and plant proteins are incomplete.
4 – LIMITING AMINOACID: of a protein or protein mixture it is the essential or lacking essential aminoacid that limits the use of all the other amino acids even if present in excess with respect to the needs. As we have seen in the proteins of vegetable origin this aminoacid is not generally sufficient to guarantee the needs and must be introduced through the combination with other foods.
5 – CHEMICAL INDEX: is given by the ratio between the quantity of a given aminoacid in a gram of the protein under examination and the quantity of the same aminoacid in a gram of the biological reference protein (of the egg). The higher this index, the greater the percentage of essential amino-acids.
6 – BRANCHED amino-acids: o BCAAs are three essential amino-acids (Valine, Isoleucine and Leucine) that under special conditions, such as intense physical effort, are used as an auxiliary energy substrate of fats and carbohydrates.
7 – Some amino-acids are also precursors of compounds that perform important biological functions. From the tryptophan niacin (vitamin PP), serotonin (neurotransmitter) and melatonin (regulator of the circadian rhythms sleep / wake cycle) are obtained.
Glutathione is obtained from sulfur amino acids (methionine and cysteine), an important antioxidant useful for combating free radicals and keratin, an essential protein for the health of hair, hair and nails. In addition to those involved in protein synthesis, many other amino-acids perform very important functions. Among these the most known in the sports field are creatine (useful for increasing capacity and anaerobic alattacid and lactic acid power) and carnitine which facilitates the transport of lipids within the mitochondria).
Back
