Gliadin and Glutenin
They are the wheat proteins (gliadin, soluble in alcohol and glutenin, insoluble in alcohol) and are composed of amino acid chains (1). Gliadin is made up of the union of about 100-200 amino acids (the main cause of celiac disease), and glutenin, consisting of a combination of about 2,000-20,000 amino acids. The covalent bond that unites two amino acids also takes the name in biochemistry of “peptide bond”. A chain of multiple amino acids linked through peptide bonds takes the generic name of peptide or polypeptide or oligopeptide if the number of amino acids involved is limited; one or more polypeptides, sometimes accompanied by other auxiliary structures or ions called cofactors or prosthetic groups, constitute a protein. amino-acids (or amminoacids) are the primary structural unit of proteins. We can therefore imagine the amino acids as bricks that, united by a glue called peptide bond, form a long sequence that gives rise to a protein. Alcohol soluble cereal proteins are called: prolamines.
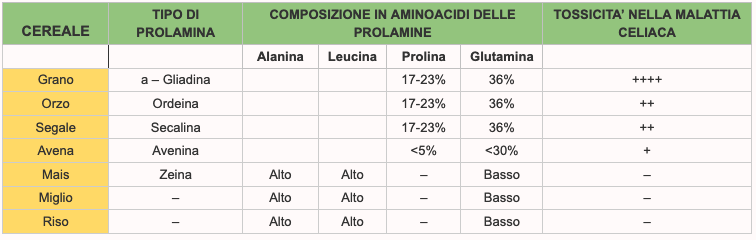
The wheat prolamine is gliadin, that of barley is hordein; that of rye is secaline, that of avena is avenin. The different types of prolamins contain different amino-acids and the higher the content of proline and glutamine (which are some of the amino-acids that compose it) the more the prolamine, and therefore the peptides of that cereal will be toxic (2) for the affected patient from celiac disease. The highest levels of proline and glutamine are in wheat, barley and rye. Also glutenins have some toxic sequences for celiacs but they appear to be much less active in soliciting the adverse response of the humanitarian system of man.