The research showed, through tests to make bread and biscuits, the best varieties of monococcum wheat among the 24 examined: among these the varieties ID140, ID280 and Id361 were the best for both uses. The research also shows how, due to the rheological properties of the flours, the presence or absence of a very limited number of storage protein subunits is important, highlighting the importance of LMW
Einkorn Characterization for Bread and Cookie Production in Relation to Protein Subunit Composition M. Corbellini, S. Empilli, P. Vaccino, A. Brandolini, B. Borghi, M. Heun, and F. Salamini. Cereal Chem. 76(5):727–733
Abstract
“Twenty-four einkorns were evaluated for agronomic traits in Italy and in Germany in replicated plot trials. After dehulling and milling, the harvested kernels, flour protein content, sedimentation volume, falling number, carotenoid, and dry gluten content were determined. Farinograph profiles were obtained with a farinograph and baking and cookie quality were evaluated with standard microtests. Significant differences in yield potential were observed between the two locations, with a three-fold increase in Germany as compared with Italy. One of the einkorn lines (ID529) had farinograph stability and degree of softening indices better than those of the control bread wheat. All the samples analyzed for breadmaking aptitude showed some degree of stickiness, but it was possible to handle the dough during the different steps of breadmaking. On average, cookies produced with einkorn flour were larger in diameter and thinner than those produced with soft wheat flour. The composition in α, β and γ-gliadins and in high molecular weight glutenin subunits was similar in all the lines. In contrast, the pattern exhibited in low molecular weight glutenin subunits correlated strictly with baking quality. In particular, the lines with bands arbitrarily designated a and b showed a high breadmaking poten- tial, while the lines lacking these bands had an ample range of variability but, on average, a much lower baking potential. Our data point to a simple genetic control of the breadmaking aptitude and indicate einkorn not only as a promising source of specialty foods but also as an ideal species for genetic investigations on wheat quality”.
NOTE:
LMW-GS: Low Molecular Weight – Glutenin Subunit
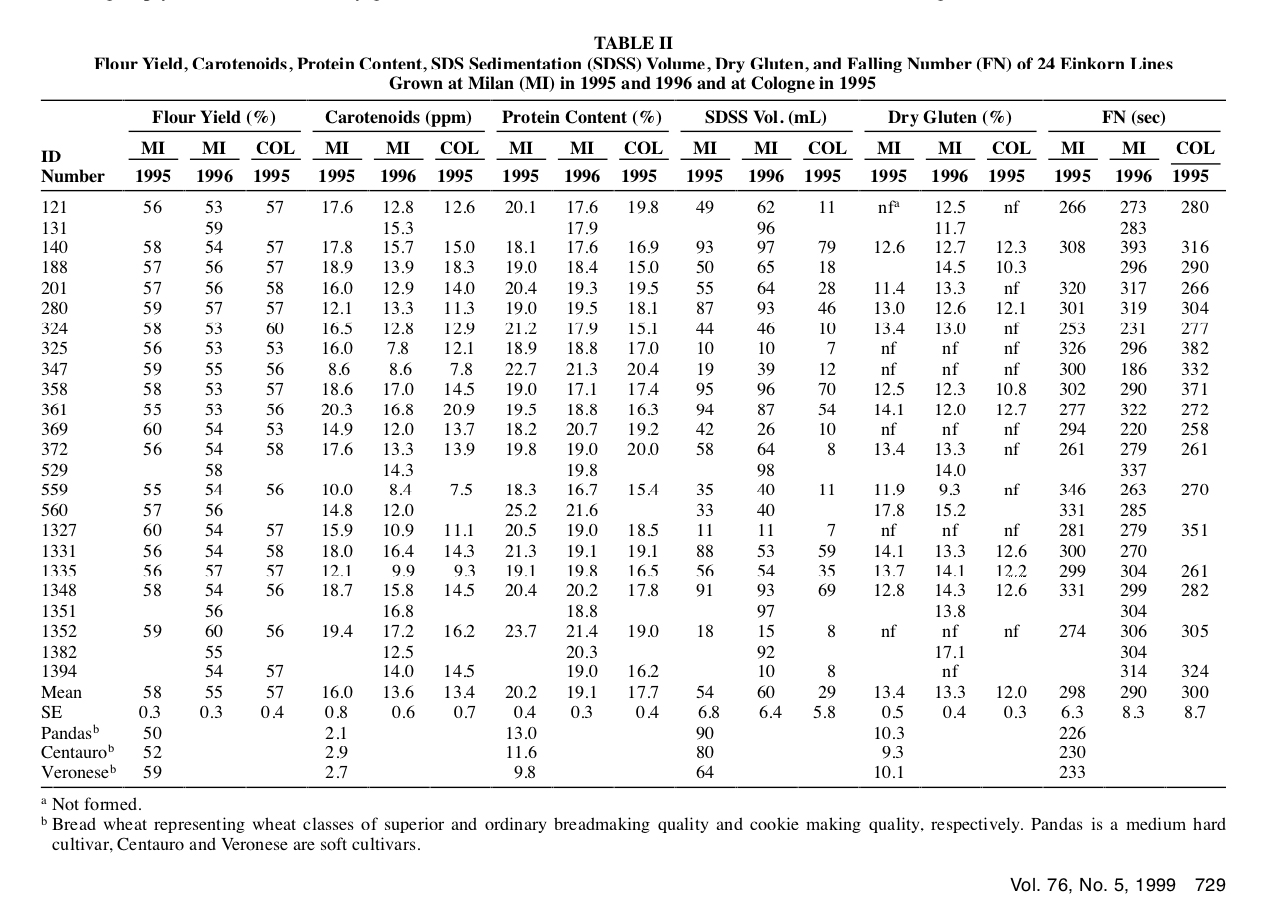
(Table extracted from the research)
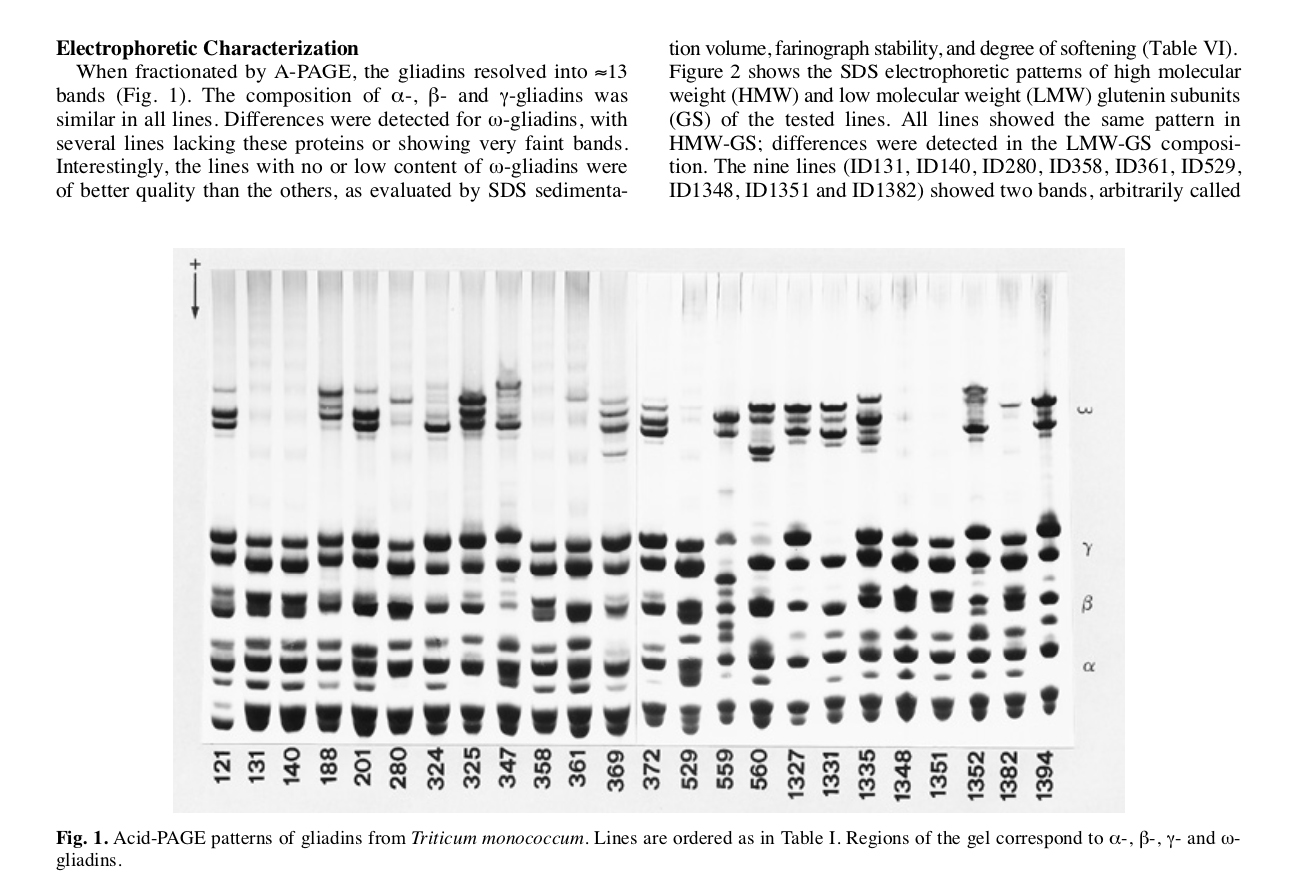
Electrophoretic characterization of reserve proteins: glutenins and gliadins. They represent, with the different respective bands, the genetic imprint that defines and identifies the variety. (Table extracted from the research)