An opportunity to be seized: digestible and tolerable gluten. Why?
An opportunity to be seized: digestible and tolerable gluten. Why?
Gluten (it is a protein compound that is formed when glutenin and gliadin, present in flour, are strongly mixed with water) is responsible for celiac disease in genetically predisposed subjects. Not all gluten is at the origin of this pathology: the research has, in fact, isolated some sequences of amino acids (they are the “bricks” that constitute gluten) that are responsible for the adverse reaction of the innate and adaptive human immune system. These sequences are present (even several times) in the molecular chains (peptides) that constitute gluten, and, above all in gliadins. There are many studies that aim to create grains or flours without these sequences, mixtures where the action of particular bacteria present in the acid paste destroy the toxic fractions. Particular enzymes (proteases produced by Aspergillus) have been identified that can activate a complete enzymatic digestion of gliadin, reducing or eliminating the reactive response of gluten-sensitive T cells. (Toft-Hansen H et al Clin Immunol. 2014 Aug; 153 (2): 323-31. Doi: 10.1016 / j.clim.2014.05.009. Epub 2014 Jun 3).
Gluten is indigestible as such, only if divided into constituent amino acids it can be digested and, after being passed into the blood, be assimilated. The action of “chopping up the gluten is carried out by the enzyme pepsin (it is the most important of the digestive enzymes and, activated by hydrochloric acid, attacks proteins and breaks them down into fragments called polypeptides which will then be broken down into individual amino acids by trypsin ), present in the stomach and the enzyme trypsin produced by the pancreas present in the intestine. These two enzymes are not always able to “break up” the gluten and the residues are eliminated by “normal” people. These residues, on the other hand, if they contain toxic sequences activate the response of the immune system that fights them as “enemies”. The more gluten is strong (ie the stronger the bonds of the molecules that make up gluten) the more difficult and the action of enzymes will be longer. You can be born celiac but you can also become genetically predisposed. At greater risk, of course, are the relatives and relatives of celiacs. Scientific research has shown that the use in the diet of foods produced with grains as light as possible and tolerable (with the least possible amount of “toxic epitopes”) reduces the possibility of becoming celiac and is indicated for non-celiac gluten sensitive people. An example regarding the monococcus wheat we find in the study:
“…..Conclusions: Our study shows that Tm (Grano Monococco) is toxic for CD patients as judged on histological and serological criteria, but it was well tolerated by the majority of patients, suggesting that Tm is not a safe cereal for celiacs, but that it may be of value for patients with gluten sensitivity or for prevention of CD.Copyright of European Journal of Nutrition is the property of Springer Science & Business Media B.V. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use. This abstract may be abridged. No warranty is given about the accuracy of the copy. Users should refer to the original published version of the material for the full abstract.”
For some time now, scientific research has highlighted another gluten-related disease: non-celiac gluten sensitivity (NCGS). Today it is possible to diagnose it only through a long and complex series of analyzes which, for this reason, cannot be widely applied. The research (well summarized in the attached research) is still on the high seas, in fact, in the realization of biomarkers suitable to diagnose this pathology in a certain and simple way. Finally it should be noted that although there are very many studies, researches and tests on patients, these have proved too partial to be able to define “with certainty” how the NCGS is activated. Gliadins, however, play an important role as anti-gliadin antigen has been found in patients diagnosed with this disease. Finally, the research showed that a light and tolerable gluten is less invasive for those with irritable bowel disease.
“During the intervention period with ancient wheat products, patients experienced a significant decrease in the severity of IBS symptoms, such as abdominal pain (P< 0·0001), bloating (P= 0·004), satisfaction with stool consistency (P< 0·001) and tiredness (P< 0·0001). No significant difference was observed after the intervention period with modern wheat products. Similarly, patients reported significant amelioration in the severity of gastrointestinal symptoms only after the ancient wheat intervention period, as measured by the intensity of pain (P= 0·001), the frequency of pain (P< 0·0001), bloating (P< 0·0001), abdominal distension (P< 0·001) and the quality of life (P< 0·0001). Interestingly, the inflammatory profile showed a significant reduction in the circulating levels of pro-inflammatory cytokines, including IL-6, IL-17, interferon-γ, monocyte chemotactic protein-1 and vascular endothelial growth factor after the intervention period with ancient wheat products, but not after the control period. (Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: a double-blinded randomised dietary intervention trial. Francesco Sofi et altri 2014.”
Therefore, although there are genetically modified grains with less toxicity, detoxified flours, pill containing enzymes that hydrolyze gluten, because “primarily” do not use ancient grains, many of which are more digestible (gluten less strong and more easily hydrolysable by digestive enzymes ) and tolerable (with a lower quantity of toxic epitopes)? Is it not an alternative, an opportunity to be seized? Of course the market, with very little information transparency, does not help: it is somewhat difficult, if not impossible, to still have information on the type of wheat, flour or products with regard to their digestibility and tolerability. An identity card would be needed with the transcription of all the passages. Regarding the digestibility of a flour, an index that defines it is the Gluten Index (gluten index). This index tells us how strong gluten is, that is how strong are the bonds of the molecules that compose it. It is not reported in any package of flour, wheat, pasta or products. Some research has highlighted the differences that exist, for example between modern grains (with exceptions) and ancient grains (not all) in relation to the strength of gluten:
“A marked improvement in gluten strength was also observed in the modern genotypes which showed a mean GI value of 50% vs 8.5% of the old genotypes under the low nitrogen input applied. This result confirmed the increase in pasta-making quality during the 20th century in durum wheat cultivars reported by De Vita et al. (2007) for Italian cultivars and by Subira et al. (2014) who found increases in gluten strength of 32.1% (0.54% year−1) and 27.9% (0.33% year−1) in Italian and Spanish cultivars, respectively. This improvement in gluten strength was attributed to the incorporation of favorable alleles in modern cultivars, such as the 7+8 HMW-GS encoded by the Glu-B1 locus (De Vita et al., 2007) and to the increased frequency of the LMW-GS combination aaa, which was present in 75% of all intermediate cultivars and in 100% of the modern Italian cultivars (Subira et al., 2014). Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy . Michele A. De Santis, Marcella M. Giuliani, Luigia Giuzio, Pasquale De Vita, Alison Lovegrove, Peter R. Shewry, Zina Flagella. European Journal of Agronomy 87 (2017) 19–29.”
From another study
“3.2.1. Gluten index
Analysis of variance showed a significant effect of the G x Y (genotype x year) interaction on the gluten index, as shown in Figure 22. Gluten index was significantly higher in the modern group of genotypes (P < 0.001). The old genotypes (from 1900 to 1949) showed a very low gluten index (min 5, max 13) without significant differences, while in the modern group of durum wheat genotypes G.I ranged from 30 to 80. Among these genotypes Claudio and Saragolla showed the highest values and Preco the lowest. The effect of the crop season was significant only in three modern cultivars (Adamello, Preco, PR22D89), resulting in higher values in 2013 crop year, characterized by water deficit during grain maturation. The highest G.I. values were measured in modern cv Saragolla in both crop seasons, while the lowest in Timilia RB and Garigliano G.I. (5).
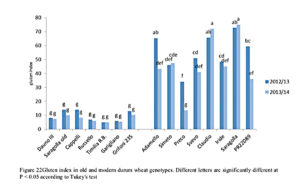
Figure 22. Gluten index in old and modern durum wheat genotypes. Different letters are significantly different at P < 0.05 according to Tukey’s test. Characterization of old and modern durum wheat genotypes in relation to gluten protein and dietary fibre composition. Phd thesis De Santis Michele. Tesi di dottorato. Università di Foggia 2016”
Other research has focused on the differences in relation to the quantity and quality of toxic fractions (toxic epitopes): “… 76 genotypes of Triticum durum underwent digestion in vitro. Peptides containing sequences known to trigger the celiac response were identified and quantified by liquid chromatography with mass spectrometry. Six different genotypes of T. durum, grown in 4 different Italian regions, were also analyzed to assess the influence of the environment on the production of peptides involved in celiac disease. 11 peptides containing sequences known to be involved in the celiac response were identified. The quantity of immunogenic peptides is very variable among the different varieties of wheat, going from 386 ppm of Valerio to 1661 ppm of Amedeo. Of the 76 genotypes analyzed, 10 produce less immunogenic peptides than 500 ppm, while 4 produce more than 1550 ppm. As shown by the data, the variability (naturally high) in the production of immunogenic peptides can be exploited to reduce the exposure of genetically predisposed subjects. Identification of low-impact wheat varieties on subjects genetically predisposed to celiac disease, for the development of food products capable of preventing their occurrence. Fatma Boukid, Barbara Prandi, Arnaldo Dossena Stefano Sforza. Interdepartmental Center SITEIA.PARMA, University of Parma, Parco Area delle Scienze, 43124, Parma, Italy; Department of Food and Drug Sciences, University of Parma, Area Science Park 17 / A, 43124, Parma, Italy Other search:
“To analyzewhether wheat breeding contributed to the increase of the prevalence of CD, we have compared the genetic diversity of gluten proteins for the presence of two CD epitopes (Glia-9 and Glia-20) in 36 modern European whea varieties and in 50 landraces representing the wheat varieties grown up to around a century ago. Glia-9 is a major (immunodominant) epitope that is recognized by the majority of CD patients. The minor Glia-20 was included as a technical reference. Overall, the presence of the Glia-9epitope was higher in the modern varieties, whereas thepresence of the Glia-20 epitope was lower, as compared to the landraces. This suggests that modern wheat breedingpractices may have led to an increased exposure to CD epitopes. On the other hand, some modern varieties and landraces have been identified that have relatively low contents of both epitopes. Such selected lines may serve as a start to breed wheat for the introduction of ‘low CD toxic’ as a new breeding trait. Large-scale culture and consumption of such varieties would considerably aid in decreasing the prevalence of CD.
In wheat, gluten proteins comprised gliadins and glutenins, which are present in approximately equal amounts andform 80% of the total storage protein content in the wheatkernel, next to albumins (12%) and globulins (8%). The gliadins form a large protein family in which α /β , γ and ω-gliadins can be distinguished (Woychik et al. 1961), whereas the glutenins can be subdivided into low-molecular weight glutenin subunits (LMW-GS) and high-molecular weight glutenin subunits (HMW-GS) (Shewry andTatham 1999). The high proline and glutamine contentmakes gluten proteins resistant to complete proteolytic digestion (Hausch et al. 2002; Shan et al. 2002, 2004). Gluten peptides resulting from partial digestion of all gluten protein groups (α /β , γ and ω-gliadins), LMW-GS, and HMW-GS) may contain T-cell stimulatory epitopes (Koning 2008;Stepniak et al. 2008), but the epitopes from the α –gliadins are considered to have by far the highest clinical relevance with regard to both the adaptive immune response and the innate immune response that lead to the development of CD (Sjöström et al. 1998; Arentz-Hansen et al. 2000a, b, 2002;Anderson et al. 2000; Janatuinen et al. 2002; Vader et al.2002; Maiuri et al. 2003; Molberg et al. 2003; Schuppanet al. 2003; Qiao et al. 2005; Marti et al. 2005; Camarcaet al. 2009). Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: wheat breeding may have contributed to increased prevalence of celiac diseaseHetty C. van den Broeck · Hein C. de Jong · Elma M. J. Salentijn · Liesbeth Dekking · Dirk Bosch · Rob J. Hamer · Ludovicus J. W. J. Gilissen · Ingrid M. van der Meer · Marinus J. M. Smulders Received: 13 April 2010 / Accepted: 25 June 2010 / Published online: 28 July 201”
Note: Enzyme based tablets: Among the latest news is a study released during Digestive Disease Week, the largest international conference dedicated to gastroenterology: Julia König and her colleagues have documented that an enzyme-based tablet to be taken at mealtimes that contain gluten can cause a large part of the protein to not reach the small intestine. These enzymes, the researchers point out, are already on the market in the United States in the form of integrators (2017). The genetic predisposition is conferred by the presence of the HLA-DQ2 and / or HLA DQ8 genes. In individuals with predisposing genes, gluten can trigger an immune reaction with consequent production of auto-antibodies and inflammatory molecules (cytokines) that damage the intestinal mucosa (atrophy of the villi, infiltration of the mucosa with inflammatory cells), altering the permeability of the intestine. NCGS and markers: “There are no specific markers of disease. The serological data that most correlates with this condition is constituted by anti-gliadin antibodies of the IgG class (AGA-IgG), found in about 50% of adult subjects and in 60% of pediatric subjects; these antibodies, while representing an element of diagnostic support, are not specific for NCGS and can also be found in celiac disease, autoimmune liver diseases, IBS, connective tissue diseases and even in healthy controls. The search for HLA-DQ2 and HLA-DQ8 (genetic predisposition haplotypes to celiac disease, positive in almost all cases in celiac disease) is positive in about 50% of the affections, with a percentage still greater than the general population; the possible involvement of adaptive immunity through the MHC complexes in the NCGS is not yet clarified. Non-celiac gluten sensitivity: a new clinical entity. Carlo Polloni, Teresa Benuzzi, Valentina Bortolotti, Erica Dal Bon, Francesca Sorrentino. Unit of Pediatrics, Santa Maria del Carmine Hospital, Rovereto (TN) School of Specialization in Pediatrics University of Verona, ”
The FODMAP content of sourdough bread can be reduced by up to 90% by utilizing a specific sourdough system. Ziegler, J.; Steiner, D.; Longin, C.; Friedrich, H.; Würschum, T.; Schweiggert, R.; Carle, R. Wheat and the irritable bowel syndrome—FODMAP levels of modern and ancient species and their retention during bread making. J. Funct. Foods 2016, 25, 257–266.
The toxic mechanism
When gliadin reaches the intestinal wall, peptic digestion has then already taken place. Further digestion by pancreatic enzymes does not destroy the toxicity of gliadin although this digestion is never complete. Some protein is stili intact and can be transported through the intestinal lining. Experimental work has demonstrated that some changes in the transported molecules take place in mice [12]. In normal circumstances we develop a tolerance for proteins in our food. Whether the enzyme system of coeliac patients has a missing component or whether there is a failure of the normal tolerance is uncertain. Cornell [13] found that a homogenate of biopsies from normal subjects digest gliadin to the level that it does not damage rat liver lysosomes, whereas a digest from a homogenate of biopsies from coeliac patients causes such a lysosomal damage. He could isolate a peptide from the coeliac incubation mixture that was not found in the normal controls. A transglutaminase – an enzyme that can use gliadin as a substrate – was also found to be lacking in the brushborder of coeliac patients, even when they had a normalised intestinal architecture on a glu-tenfreediet [14].
The way of presentation of gliadin fragments or their handling during transport can be the first step in the reaction to gliadin in the enterocytic lining the intestinal wall. Passage of insufficiently digested peptides or break of tolerance then brings the immune system into action. Apart from the formation of antibodies, it is the cellular immune system in particular that reacts to the peptides that are presented to them [15]. This immune reaction in turn is an important factor in the structural and functional changes of the intestinal celi lining. More protein or peptide is able to pass through the brush border layer of the enterocytes, leading to mucosal atrophy in coeliac patients.
Gliadin toxicity is not a quantitative problem. From the Dutch food consumption survey 1987-1988 [18] the normal consumption of gliadin for the different age groups in the Netherlands can be calculated. For children from 2-5 years this amount is 200-250 mg/kg/day; for adults it is about 100 mg/kg/day. Tests with much higher quantities of gluten did not result in villus atrophy in normal individuals, so the effect is not due to differences in sensitivity between normal and CD patients. William Th. J.M. Hekkens Department ofPhysiology, Medicai faculty, University of Leiden, PO Box 9604, 2300 RC Leiden, The Netherlands
The use of natural yeast containing fungal enzymes and selected lactic bacteria – explains Gobbetti – is able to completely degrade gluten during the food transformation process. In this way the residual gluten content is less than ’10 ppm’, well below the maximum threshold allowed for gluten-free foods “. Gluten, therefore,” is not masked but is completely degraded into fragments that are no longer able to cause intolerance “.DA: University, in Bari realized the first wheat bread without gluten (Giuliani SPA detoxified flour). Tuesday 5 May 2015. ttp: //www.quotidianodipuglia.it/scuola_e_universita/universita_bari_pane_senza_glutine-1016808.html.
Depeening
Recent advances in understanding non-celiac gluten sensitivity
Back
