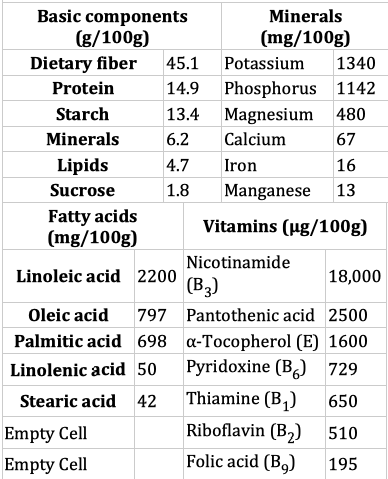
Highlighted:
1 – In gluten-free products it was founded nutrient deficiencies for essential minerals such as iron, zinc magnesium and calcium, and on another hand high content of saturated lipids were detected Industrial gluten-free products in many cases contain palm and palm kernel oil
2 – Despite improvements in the formulation of GFPs in recent years, their macronutrient profile suggested they contained marked differences and cannot be considered nutritionally equivalent when compared with their gluten-containing counterparts
3 – Good fats include monounsaturated and polyunsaturated fats. Bad ones include industrial-made trans fats. Saturated fats fall somewhere in the middle
4 – In 2015, the FDATrusted Source declared that trans fat is not “generally recognized as safe” and had to be phased out by 2018.
5 -A recent study, published in The Lancet Diabetes & Endocrinology, evaluated for the first time the association between emulsifiers and the risk of developing type 2 diabete
A – Nutritional quality and costs of gluten-free products: a case-control study of food products on the Norwegian marked. 2021. Mari C. W. Myhrstad, Marlene Slydahl, Monica Hellmann, Lisa Garnweidner-Holme, Knut E. A. Lundin et al.
Background: Celiac disease is a chronic autoimmune disease triggered by gluten exposure in genetically predis- posed individuals. A life-long intake of a gluten-free (GF) diet is required for its management. Wheat, rye and barley are eliminated in a GF diet and the nutritional adequacy of the diet has been questioned. In Norway, cereals and bread constitute a key role of the diet and are the main source of fiber intake. Gluten restrictions may therefore offer important implications for nutrient adequacy especially linked to fiber intake in people with celiac disease.
Objective: The aim of the study was to investigate the nutritional quality and price of GF products and com- pare with gluten-containing counterparts available at instead of in the Norwegian market.
….omissis
The current study clearly shows that GF products compared to equivalent gluten-containing products contain less protein and fiber, and more carbohydrate, saturated fat and salt. Furthermore, GF compared to gluten-containing products are more expensive. To our knowledge, this is the first study comparing GF products at the Norwegian market with gluten-containing counterparts.
@@@@
B – Review on chemical composition of gluten-free food for celiac people. Antonella Maggio, Santino Orecchio and Salvatore Barreca. Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Università di Palermo, Viale delle Scienze, I-90128 Palermo, Italy. Integr Food Nutr Metab, 2019. Published: January 25, 2019.
Abtract: Gluten free food lead to possible nutrient unbalance resulting in improper nutritional quality of diet. The aim of this review is to show and discuss the composition of main components of common gluten free products in order to provide doctors and nutritionists the necessary data to compile balanced diets for users of gluten-free products and to determine their contribution to the daily intake of nutrients and micro elements. Special emphasis has been addressed to metal contents, fatty acid profiles and fibers.
……omissis
Conclusions
Most of the nutritional data reported in literature, are based on food labels. Few data were obtained by direct chemical analysis of food. In this context, will be necessary to encourage the use of chemical analytical practices in order to provide doctors and nutritionists the necessary data to compile balanced diets for users of gluten-free products and to determine their contribution to the daily intake of nutrients and micro elements. Special emphasis has been addressed to metal contents, fatty acid profiles and fibers.
Literature analysis has highlighted that, the most gluten free food, show a deficit of nutrients in term of concentrations. At this regard, an inadequate nutritional value of the GF-diet was observed from several authors. In detail, it was founded nutrient deficiencies for essential minerals such as iron, zinc magnesium and calcium, and on another hand high content of saturated lipids were detected.
Furthermore, the dietary-therapeutic approach should encourage the use of naturally gluten free products such as pseudo-cereals and fruits concerning to metal contents, and fish or seafood regarding fatty acids, especially for sutured and unsatured fatty acid ratio.
Moreover, alimentary education should become part of the therapeutic pathway to understand the importance of labels, choice of food and combination of macro and micronutrients.
@@@@@@
C – Fatty Acid Composition of Gluten-Free Food (Bakery Products) for Celiac People. Antonella Maggio and Santino Orecchio. Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Università di Palermo, Viale delle Scienze, I-90128 Palermo, Italy; antonella.maggio@unipa.it. Correspondence: santino.orecchio@unipa.it; Tel.: +39-91-2389-7968. Foods. Published: 20 June 2018
Abstract: The aim of this study (first analytical approach) was to obtain data concerning the fatty acid composition of gluten-free foods (bakery products) for celiac people. The study included 35 different products (snacks, biscuits, bakery products, pasta, flours, etc.) from several manufacturers. After extraction and esterification, the fatty acid composition was determined by Gaschromatography (GC–MS) Monounsaturated fatty acids (MUFAs) were found to be the major constituents (57%), followed by saturated fatty acids (SFAs) (30%), and polyunsaturated fatty acid (13%). Only 15 of the 35 gluten-free samples analyzed appeared to provide adequate energy intake, while, in 11 samples, saturated fatty acids were found to supply more energy than that recommended by the European Food Safety Authority EFSA. Moreover, data analyses showed that, although gluten-free commercial products are high added-value foods, industrial products in many cases contain palm and palm kernel oils, whereas the local producers generally use the finest raw materials, such as olive oil.
@@@@@@
D – Gluten-Free Products: Do We Need to Update Our Knowledge? Claudia Marmol-Soler. Foods 2022.
It can be concluded that reviewing the nutritional composition of GF foods from time to time is highly relevant since these products, which are in great demand, undergo constant changes in their composition with the aim of improving their nutritional quality. Despite improvements in the formulation of GFPs in recent years, their macronutrient profile suggested they contained marked differences and cannot be considered nutritionally equivalent when compared with their gluten-containing counterparts. Therefore, it is strongly recommended that food companies continue with the reformulation of these products in order to increase their nutritional quality, adapt to market demands, and accordingly provide balanced nutrition to those patients with CD.
@@@@@@
E – The truth about fats: the good, the bad, and the in-between
April 12, 2022. Avoid the trans fats, limit the saturated fats, and replace with essential polyunsaturated fats . Harvard Medical School. https://www.health.harvard.edu/
You may wonder isn’t fat bad for you, but your body needs some fat from food. It’s a major source of energy. It helps you absorb some vitamins and minerals. Fat is needed to build cell membranes, the vital exterior of each cell, and the sheaths surrounding nerves. It is essential for blood clotting, muscle movement, and inflammation. For long-term health, some fats are better than others. Good fats include monounsaturated and polyunsaturated fats. Bad ones include industrial-made trans fats. Saturated fats fall somewhere in the middle.
All fats have a similar chemical structure: a chain of carbon atoms bonded to hydrogen atoms. What makes one fat different from another is the length and shape of the carbon chain and the number of hydrogen atoms connected to the carbon atoms. Seemingly slight differences in structure translate into crucial differences in form and function.
Bad trans fats
The worst type of dietary fat is the kind known as trans fat. It is a byproduct of a process called hydrogenation that is used to turn healthy oils into solids and to prevent them from becoming rancid. Trans fats have no known health benefits and that there is no safe level of consumption. Therefore, they have been officially banned in the United States.
Early in the 20thcentury, trans fats were found mainly in solid margarines and vegetable shortening. As food makers learned new ways to use partially hydrogenated vegetable oils, they began appearing in everything from commercial cookies and pastries to fast-food French fries. Trans fats are now banned in the U.S. and many other countries.
Eating foods rich in trans fats increases the amount of harmful LDL cholesterol in the bloodstream and reduces the amount of beneficial HDL cholesterol. Trans fats create inflammation, which is linked to heart disease, stroke, diabetes, and other chronic conditions. They contribute to insulin resistance, which increases the risk of developing type 2 diabetes. Even small amounts of trans fats can harm health: for every 2% of calories from trans fat consumed daily, the risk of heart disease rises by 23%.
@@@@@@
F- Hydrogenated oil comes in two forms: partially or fully hydrogenated. One use of hydrogenated oil is to preserve the shelf life of food. Partially hydrogenated oil contains trans fat that can raise cholesterol and result in health complications. Food manufacturers use hydrogenated oil as a preservative. They also use it for enhancing flavor and texture.
In 2015, the Food and Drug Administration (FDA)Trusted Source said that partially hydrogenated oil is not safe, and removing it from food could prevent thousands of heart attacks each year.
Partially hydrogenated oil (trans fat)
In the past, manufacturers added partially hydrogenated oils to processed foods.
According to the FDA, foods that used to contain large amounts of artificial trans fat include:
most baked goods
stick margarine
frosting
coffee creamers
snack foods
In 2015, the FDATrusted Source declared that trans fat is not “generally recognized as safe” and had to be phased out by 2018.
However, trans fat may still be present in some foods. According to the American Heart Association (AHA)Trusted Source, trans fat occurs naturally in certain animals, such as cows.
Fully hydrogenated oil
Fully hydrogenated oil also uses a process to take a liquid oil and transform it into a solid at room temperature. As the name suggests, the oil is fully or nearly completely hydrogenated, which reduces the amount of trans fat in the final product. Unlike partially hydrogenated oil, the FDATrusted Source still allow products to use fully hydrogenated oil as of 2018. In 2020, the FDA released certification that states fully hydrogenated rapeseed oil is safe for sparing use in food products. Though hydrogenated oils may be safe, it does not mean they are necessarily good for a person to consume. Products that contain them are often highly processed with added sugar and salt.
From: https://www.medicalnewstoday.com/articles/325266#summary
@@@@@@
G- Nutritional quality and costs of gluten-free products: a case-control study of food products on the Norwegian market. Mari C.W. Myhrstad et al. 2021
Results: The GF products contained less protein and fier, and higher content of saturated fat, carbohydrate and salt compared to the gluten-containing products. The total amount of fat was not different between the groups. A similar pattern was found within several of the food categories. More gluten-containing products met the nutrition claim “high in fier” (fiber > 6 g/100 g) compared to the GF products. The price of the GF products was higher; ranging from 46%–443% more expensive than the gluten-containing products.
@@@@@
H – Macchine alimentari – Prodotti e tecnologie per alimenti senza glutine. Anno XVII -1 – Genn. Feb 2015
Idrocolloidi. (H1) Tra le ultime novità che possono soddisfare queste esigenze troviamo, come sopra citati, gli idrocolloidi, che si stanno affermando con maggiore forza nel settore bakery. Queste sostanze, infatti, permettono di ottenere prodotti con lunga shelf life, inserimento di farine integrali e fibre, l’assenza di grassi trans e non ultimo l’assenza di glutine. Gli idrocolloidi, come il termine fa presagire, sono molecole in grado di legare acqua in grandi quantità; tra i più usati nei prodotti da forno vi sono la gomma di xantano, la pectina, le cellulose modificate e i frutto- e galatto-oligosaccaridi. Alcune di queste sostanze sono considerate fibre alimentari, in grado di stimolare il senso di sazietà e avere effetti positivi sulla funzionalità intestinale: la loro presenza si può configurare, pertanto, co- me aggiunta di sostanze benefiche al prodotto. Spesso gli idrocolloidi ottengono il loro effetto tecnologico-funzionale nel prodotto anche se aggiunti agli impasti in piccole quantità, per esempio minori dell’1% del totale degli ingredienti in polvere. Negli impasti di pane e altri prodotti da forno gli idrocolloidi aiutano, in fase produttiva, a migliorare la lavorabilità dell’impasto grazie all’effetto di rapida ed uniforme idratazione dello stesso. Il volume, la struttura e la sofficità dei prodotti finiti sono migliorati. La fragilità è minore, per esempio nel caso di prodotti da forno “spumosi” con elevata presenza di bolle d’aria o presenza di pezzi in sospensione (cioccolato, frutta o frutta secca): tali bolle o pezzi sono stabilizzati all’interno del sistema grazie agli idrocolloidi. In fase di conservazione, poi, c’è un aumento della shelf life dei prodotti grazie al mantenimento di sofficità per tempi più prolungati: la differenza rispetto ai prodotti privi di idrocolloidi è tanto più evidente con il passare del tempo. Pare, infine, che la presenza di idrocolloidi sia anche in grado di influenzare le dimensioni dei cristalli di ghiaccio all’interno degli impasti per pane o altri prodotti semi-cotti durante la loro surgelazione, permettendo di ottenere un prodotto scongelato di migliore qualità.
Omissis…
(H2) Ci sono operazioni unitarie che sono di difficile attuazione per alimenti che non prevedono l’uso di glutine, come per esempio le fasi di estrusione, trafilatura o laminazione che avvengono nella pasta oppure in alcuni prodotti da forno: le sollecitazioni che avvengono in queste fasi necessitano di elasticità da parte dell’impasto, pertanto sono fondamentali formulazioni in grado di sostenere il processo in continuo di un impianto magari pre-esistente.
Omissis….
(H3) Se si confrontano dei cracker senza glutine, si riscontrano formulazioni estremamente semplici, con farine di mais e riso, ed altre più complesse, con l’aggiunta di fecola di patate, destrosio, emulsionanti ed addensanti. Dal punto di vista nutrizionale, è chiaro che l’alimento potrebbe risultare, rispetto al medesimo prodotto convenzionale, maggiormente ricco di zuccheri ed in parte di grassi. Il pane in cassetta, più difficile da realizzare in quanto lievitato, mostra formulazioni piuttosto complesse a base di mais, riso o grano saraceno, amidi, fibre vegetali, proteine, zuccheri, addensanti (tra cui idrocolloidi), emulsionanti, acidificanti. Tale ricettazione implica, a livello nutrizionale, o un aumento di carboidrati di circa il 10- 15% rispetto al prodotto convenzionale della medesima categoria oppure un aumento di grassi, soprattutto saturi, di circa il 30-50%.
Nel campo dolciario, le considerazioni sono più o meno le medesime, in quanto a livello nutrizio- nale, rispetto ai prodotti convenzionali, permangono valori più elevati di carboidrati, soprattutto zuccheri, e grassi, principalmente saturi, per sop- perire alla carenza di viscoelasticità della parte proteica.
@@@@@
I – Emulsionanti e rischio diabete: lo studio di Lancet
Dopo essere stati accusati di contribuire al rischio di obesità, cancro e malattie cardiovascolari, un’analisi recente condotta sullo studio prospettico di coorte NutriNet Santé li identifica come fattori che aumentano il rischio di diabete di tipo 2.
Sebbene le Autorità Sanitarie considerino sicuro il loro uso in quantità definite, basandosi su criteri di citotossicità e genotossicità, di recente stanno emergendo prove dei loro effetti negativi sul microbiota intestinale, che a sua volta innescano infiammazione e alterazioni metaboliche.
Un recente studio, pubblicato su The Lancet Diabetes & Endocrinology ha valutato per la prima volta l’associazione tra emulsionanti e rischio di sviluppare diabete di tipo 2. Gli Autori hanno analizzato i dati di oltre 104 mila adulti arruolati dal 2009 al 2023 a cui è stato chiesto di compilare registri dietetici di 24 ore ogni 6 mesi. L’obiettivo era valutare l’esposizione agli emulsionanti.
L’1% del campione, ha sviluppato diabete di tipo 2 durante il follow up di 6-8 anni.
Dei 61 additivi identificati, sono sette gli emulsionanti ‘attenzionati’ associati a un potenziale aumento del rischio di diabete (occhi, quindi, alle etichette!):
E407 (carragenine totali);
E340 (esteri di poliglicerolo);
E472e (esteri di acidi grassi);
E331 (citrato di sodio);
E412 (gomma di guar);
E414 (gomma arabica);
E415 (gomma di xantano);
oltre ad un gruppo chiamato ‘carragenine’.
Gli additivi emulsionanti sono stati assunti nel 5% da frutta e verdure ultra lavorate (come verdure in scatola e frutta sciroppata), nel 14.7% da torte e biscotti, nel 10% da prodotti lattiero-caseari.
Tre conseguenze sottolineate dal prof. Angelo Avogaro, Presidente SID
1. La necessità di contenere il consumo di cibi ultra-processati;
2. l’appello a una maggiore attenzione alle etichette;
3. la necessità di chiedere una regolamentazione più stringente allo scopo di proteggere i consumatori.
“Sebbene siano necessari ulteriori studi a lungo termine, le alterazioni del microbiota intestinale, fanno ritenere che potrebbe essere necessario rivedere gli RDA (Recommended Daily Allowance, livelli giornalieri di assunzione). Precedenti prove che legavano l’assunzione di carragenina all’infiammazione intestinale hanno portato l’JECFA a limitarne l’uso nelle formule e negli alimenti per neonati. Stiamo assistendo a un preoccupante aumento del diabete di tipo 2 anche tra bambini e adolescenti” sottolinea la Prof.ssa Raffaella Buzzetti, Presidente eletto SID.
References
Food additive emulsifiers and the risk of type 2 diabetes: analysis of data from the NutriNet-Santé prospective cohort study. The Lancet Diabete and Endocrinology, volume 12, issue 5, p339-349, May 2024